Abstract
Introduction
Fatigue is the most common and debilitating symptom experienced by cancer patients undergoing chemotherapy (CTX). Prediction of symptom severity can assist clinicians to identify high-risk patients and provide education to decrease symptom severity. The purpose of this study was to predict the severity of morning fatigue in the week following the administration of CTX.
Methods
Outpatients (n = 1217) completed questionnaires 1 week prior to and 1 week following administration of CTX. Morning fatigue was measured using the Lee Fatigue Scale (LFS). Separate prediction models for morning fatigue severity were created using 157 demographic, clinical, symptom, and psychosocial adjustment characteristics and either morning fatigue scores or individual fatigue item scores. Prediction models were created using two regression and five machine learning approaches.
Results
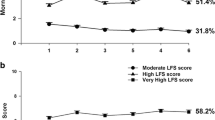
Elastic net models provided the best fit across all models. For the EN model using individual LFS item scores, two of the 13 individual LFS items (i.e., “worn out,” “exhausted”) were the strongest predictors.
Conclusions
This study is the first to use machine learning techniques to accurately predict the severity of morning fatigue from prior to through the week following the administration of CTX using total and individual item scores from the Lee Fatigue Scale (LFS). Our findings suggest that the language used to assess clinical fatigue in oncology patients is important and that two simple questions may be used to predict morning fatigue severity.


Similar content being viewed by others
References
Papadakos JK, Charow RC, Papadakos CJ, Moody LJ, Giuliani ME (2019) Evaluating cancer patient-reported outcome measures: readability and implications for clinical use. Cancer 125(8):1350–1356
Kotronoulas G, Kearney N, Maguire R, Harrow A, Di Domenico D, Croy S, MacGillivray S (2014) What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol 32(14):1480–1501
Lipscomb J, Reeve BB, Clauser SB, Abrams JS, Bruner DW, Burke LB, Denicoff AM, Ganz PA, Gondek K, Minasian LM, O’Mara AM, Revicki DA, Rock EP, Rowland JH, Sgambati M, Trimble EL (2007) Patient-reported outcomes assessment in cancer trials: taking stock, moving forward. J Clin Oncol 25(32):5133–5140
Chen J, Ou L, Hollis SJ (2013) A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res 13:211
Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, Schrag D (2017) Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 318(2):197–198
Al Maqbali M, Al Sinani M, Al Naamani Z, Al Badi K, Tanash MI (2021) Prevalence of fatigue in patients with cancer: a systematic review and meta-analysis. J Pain Symptom Manage 61(1):167–189 e114
D’Silva F, Javeth A, Singh P (2022) Cancer-related fatigue - clinical evaluation scales and interventions: a systematic review. Indian. J Palliat Care 28(1):88–98
Al Maqbali M (2021) Cancer-related fatigue: an overview. Br J Nurs 30(4):S36–S43
Gentile D, Beeler D, Wang XS, Ben-Ayre E, Zick SM, Bao T, Carlson LE, Ghelman R, Master V, Tripathy D, Zhi WI (2022) Cancer-related fatigue outcome measures in integrative oncology: evidence for practice and research recommendations. Oncology (Williston Park) 36(5):276–287
Bower JE (2019) The role of neuro-immune interactions in cancer-related fatigue: biobehavioral risk factors and mechanisms. Cancer 125(3):353–364
Howell D, Harth T, Brown J, Bennett C, Boyko S (2017) Self-management education interventions for patients with cancer: a systematic review. Support Care Cancer 25(4):1323–1355
Dimsdale JE, Ancoli-Israel S, Ayalon L, Elsmore TF, Gruen W (2007) Taking fatigue seriously, II: variability in fatigue levels in cancer patients. Psychosomatics 48(3):247–252
Dhruva A, Aouizerat BE, Cooper B, Paul SM, Dodd M, West C, Wara W, Lee K, Dunn LB, Langford DJ, Merriman JD, Baggott C, Cataldo J, Ritchie C, Kober K, Leutwyler H, Miaskowski C (2013) Differences in morning and evening fatigue in oncology patients and their family caregivers. Eur J Oncol Nurs 17(6):841–848
Kober KM, Cooper BA, Paul SM, Dunn LB, Levine JD, Wright F, Hammer MJ, Mastick J, Venook A, Aouizerat BE, Miaskowski C (2016) Subgroups of chemotherapy patients with distinct morning and evening fatigue trajectories. Support Care Cancer 24(4):1473–1485
Wright F, Cooper BA, Conley YP, Hammer MJ, Chen LM, Paul SM, Levine JD, Miaskowski C, Kober KM (2017) Distinct evening fatigue profiles in oncology outpatients receiving chemotherapy. Fatigue 5(3):131–144
Wright F, D’Eramo Melkus G, Hammer M, Schmidt BL, Knobf MT, Paul SM, Cartwright F, Mastick J, Cooper BA, Chen LM, Melisko M, Levine JD, Kober K, Aouizerat BE, Miaskowski C (2015) Trajectories of evening fatigue in oncology outpatients receiving chemotherapy. J Pain and Symptom Manage 50(2):163–175
Wright F, D'Eramo Melkus G, Hammer M, Schmidt BL, Knobf MT, Paul SM, Cartwright F, Mastick J, Cooper BA, Chen LM, Melisko M, Levine JD, Kober K, Aouizerat BE, Miaskowski C (2015) Predictors and trajectories of morning fatigue are distinct from evening fatigue. J Pain Symptom Manage 50(2):176–189
Wright F, Dunn LB, Paul SM, Conley YP, Levine JD, Hammer MJ, Cooper BA, Miaskowski C, Kober KM (2019) Morning fatigue severity profiles in oncology outpatients receiving chemotherapy. Cancer Nurs 42(5):355–364
Wright F, Hammer M, Paul SM, Aouizerat BE, Kober KM, Conley YP, Cooper BA, Dunn LB, Levine JD, G DEM, Miaskowski C (2017) Inflammatory pathway genes associated with inter-individual variability in the trajectories of morning and evening fatigue in patients receiving chemotherapy. Cytokine 91:187–210
Wright F, Kober KM, Cooper BA, Paul SM, Conley YP, Hammer M, Levine JD, Miaskowski C (2020) Higher levels of stress and different coping strategies are associated with greater morning and evening fatigue severity in oncology patients receiving chemotherapy. Support Care Cancer 28(10):4697–4706
Molassiotis A, Chan CW (2004) Fatigue patterns in Chinese patients receiving radiotherapy. Eur J Oncol Nurs Official J Eur Oncol Nurs Soc 8(4):334–340
Jim HS, Small B, Faul LA, Franzen J, Apte S, Jacobsen PB (2011) Fatigue, depression, sleep, and activity during chemotherapy: daily and intraday variation and relationships among symptom changes. Annals of Behavioral Medicine: a Publication of the Society of. Behav Med 42(3):321–333
Bzdok D, Altman N, Krzywinski M (2018) Statistics versus machine learning. Nat Methods 15(4):233–234
Bzdok D (2017) Classical statistics and statistical learning in imaging neuroscience. Front Neurosci 11:543
Tu JV (1996) Advantages and disadvantages of using artificial neural networks versus logistic regression for predicting medical outcomes. J Clin Epidemiol 49(11):1225–1231
Aria M, Cuccurullo C, Gnasso A (2021) A comparison among interpretative proposals for random forests. Machine Learn Appl 6:100094
Rauschenberger A, Glaab E, van de Wiel MA (2020) Predictive and interpretable models via the stacked elastic net. Bioinformatics 37(14):2012–2016
Bzdok D, Krzywinski M, Altman N (2017) Points of significance: machine learning: a primer. Nat Methods 14(12):1119–1120
Kober KM, Roy R, Dhruva A, Conley YP, Chan RJ, Cooper B, Olshen A, Miaskowski C (2021) Prediction of evening fatigue severity in outpatients receiving chemotherapy: less may be more. Fatigue 9(1):14–32
Saligan LN, Fernandez-Martinez JL, deAndres-Galiana EJ, Sonis S (2014) Supervised classification by filter methods and recursive feature elimination predicts risk of radiotherapy-related fatigue in patients with prostate cancer. Cancer Inform 13:141–152
Lee KA, Hicks G, Nino-Murcia G (1991) Validity and reliability of a scale to assess fatigue. Psychiat Res 36(3):291–298
Papachristou N, Puschmann D, Barnaghi P, Cooper B, Hu X, Maguire R, Apostolidis K, Conley YP, Hammer M, Katsaragakis S, Kober KM, Levine JD, McCann L, Patiraki E, Furlong EP, Fox PA, Paul SM, Ream E, Wright F, Miaskowski C (2018) Learning from data to predict future symptoms of oncology patients. PLoS ONE 13(12):e0208808
Miaskowski C, Cooper BA, Aouizerat B, Melisko M, Chen LM, Dunn L, Hu X, Kober KM, Mastick J, Levine JD, Hammer M, Wright F, Harris J, Armes J, Furlong E, Fox P, Ream E, Maguire R, Kearney N (2017) The symptom phenotype of oncology outpatients remains relatively stable from prior to through 1 week following chemotherapy. Eur J Cancer Care (Engl) 26(3):e12437
Babor T, Higgins-Biddle J, Saunders J, Monteiro M (2001) The alcohol use disorders identification test (AUDIT): guidelines for use in primary care. World Health Organization, Geneva
Karnofsky D (1977) In: Kennealey GT, Mitchell MS (eds) Performance scale. Plenum Press, New York
Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN (2003) The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum 49(2):156–163
Kozlowski LT, Porter CQ, Orleans CT, Pope M, Heatherton T (1994) Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug Alcohol Depend 34(3):211–216
Carver CS (1997) You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med 4(1):92–100
Costa PT, McCrae RR (1989) NEO five-factor inventory (NEO-FFI). Psychological Assessment Resources, Odessa, FL, p 3
Spielberger C, Gorsuch R, Suchene R, Vagg P, Jacobs G (1983) Manual for the state-anxiety (form Y): self evaluation questionnaire. Palo Alto, CA
Radloff LS (1977) The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measure 1:385–401
Lee KA (1992) Self-reported sleep disturbances in employed women. Sleep 15(6):493–498
Cimprich B, Visovatti M, Ronis DL (2011) The Attentional Function Index--a self-report cognitive measure. Psychooncology 20(2):194–202
Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, Sobel K, Coyle N, Kemeny N, Norton L (1994) The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer 30(9):1326–1336
Cohen S, Kamarck T, Mermelstein R (1983) A global measure of perceived stress. J Health Soc Behav 24(4):385–396
Weiss DS, Marmar CR (1997) In: Wilson J, Keane TM (eds) The impact of event scale - revised. Guilford Press, New York
Herth K (1992) Abbreviated instrument to measure hope: development and psychometric evaluation. J Adv Nurs 17(10):1251–1259
Connor KM, Davidson JR (2003) Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety 18(2):76–82
Extermann M, Bonetti M, Sledge GW, O’Dwyer PJ, Bonomi P, Benson AB 3rd. (2004) MAX2--a convenient index to estimate the average per patient risk for chemotherapy toxicity; validation in ECOG trials. Eur J Cancer 40(8):1193–1198
Singh KP, Kober KM, Dhruva AA, Flowers E, Paul SM, Hammer MJ, Cartwright F, Wright F, Conley YP, Levine JD, Miaskowski C (2018) Risk factors associated with chemotherapy-induced nausea in the week before the next cycle and impact of nausea on quality of life outcomes. J Pain Symp Manage 56(3):352–362
Fletcher BS, Paul SM, Dodd MJ, Schumacher K, West C, Cooper B, Lee K, Aouizerat B, Swift P, Wara W, Miaskowski CA (2008) Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. J Clin Oncol 26(4):599–605
Team RC (2019) R: A Language And Environment For Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
Traverso A, Dankers F, Osong B, Wee L, van Kuijk SMJ (2019) Diving deeper into models. In: Kubben P, Dumontier M, Dekker A (eds) Fundamentals Of Clinical Data Science. Cham (CH), Springer, Cham, pp 121–133
Breiman L, Friedman J, Olshen R, Stone C (1984) Classification And Regression Trees. Wadsworth, New York
Breiman L (2001) Random forests. Machine Learn 45(1):5–32
Liaw A, Weiner M (2002) Classification and regression by randomForest. R News 2(3):18–22
Karatzoglou A, Smola A, Hornik K, Zeileis A (2004) Kernlab - an S4 package for kernel methods in R. J Stat Soft 11(9):1–20
Santosa F, Symes W (1986) Linear inversion of band-limited reflection seismograms. SIAM J Sci and Stat Comput 7(7):1307–1330
Tibshirani R (1996) Regression shrinkage and selection via the LASSO. J Royal Stat Soc Series B (Methodological) 58(1):267–288
Zou H, Hastie T (2005) Regularization and variable selection via the elastic net. J Royal Stat Soc: Series B (Stat Methodol) 67(2):301–320
Smialowski P, Frishman D, Kramer S (2010) Pitfalls of supervised feature selection. Bioinformatics 26(3):440–443
Dankers F, Traverso A, Wee L, van Kuijk SMJ (2019) Prediction modeling methodology. In: Kubben P, Dumontier M, Dekker A (eds) Fundamentals Of Clinical Data Science. Cham (CH), Springer, Cham, pp 101–120
Rodriguez JD, Perez A, Lozano JA (2010) Sensitivity analysis of kappa-fold cross validation in prediction error estimation. IEEE Trans Pattern Anal Mach Intell 32(3):569–575
Chai T, Draxler RR (2014) Root mean square error (RMSE) or mean absolute error (MAE)? – arguments against avoiding RMSE in the literature. Geosci Model Dev 7(3):1247–1250
Kuhn M (2008) Building predictive models in R using the caret package. J Stat Soft 28(5):26
Hauser K, Rybicki L, Walsh D (2010) What’s in a name? Word descriptors of cancer-related fatigue. Palliat Med 24(7):724–730
Wang XS, Cleeland CS, Mendoza TR, Yun YH, Wang Y, Okuyama T, Johnson VE (2010) Impact of cultural and linguistic factors on symptom reporting by patients with cancer. J Natl Cancer Inst 102(10):732–738
Radbruch L, Strasser F, Elsner F, Goncalves JF, Loge J, Kaasa S, Nauck F, Stone P (2008) Research steering committee of the european association for palliative C. Fatigue in palliative care patients -- an EAPC approach. Palliat Med 22(1):13–32
Lerdal A, Kottorp A, Gay C, Aouizerat BE, Lee KA, Miaskowski C (2016) A Rasch analysis of assessments of morning and evening fatigue in oncology patients using the Lee Fatigue Scale. J Pain Symp Manage 51(6):1002–1012
Torstveit AH, Miaskowski C, Løyland B, Grov EK, Guren MG, Ritchie CS, Paul SM, Kleven AG, Utne I (2021) Common and distinct characteristics associated with self-reported functional status in older patients with cancer receiving chemotherapy. Eur J Oncol Nurs 54:102033
Jung JY, Lee JM, Kim MS, Shim YM, Zo JI, Yun YH (2018) Comparison of fatigue, depression, and anxiety as factors affecting posttreatment health-related quality of life in lung cancer survivors. Psychooncology 27(2):465–470
Bogardus ST Jr, Towle V, Williams CS, Desai MM, Inouye SK (2001) What does the medical record reveal about functional status? A comparison of medical record and interview data. J Gen Intern Med 16(11):728–736
Newman-Griffis D, Porcino J, Zirikly A, Thieu T, Camacho Maldonado J, Ho PS, Ding M, Chan L, Rasch E (2019) Broadening horizons: the case for capturing function and the role of health informatics in its use. BMC Public Health 19(1):1288
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5(6):649–656
Mann SL, Collier WH, Ose D, Brogan S, Beck AC, Sindt JE (2018) Clinician versus patient: who gets it right when assessing function in palliative care? J Clin Oncol 36(34_suppl):40
Popovic G, Harhara T, Pope A, Al-Awamer A, Banerjee S, Bryson J, Mak E, Lau J, Hannon B, Swami N, Le LW, Zimmermann C (2018) Patient-reported functional status in outpatients with advanced cancer: correlation with physician-reported scores and survival. J Pain Symp Manage 55(6):1500–1508
Jankowski C, Carpenter KM, Aranha O, Ballinger T, Banerjee C, Berger A, Breitbart W, Chang Y, Davis E, Dest V, DuBenske LL, Escalante C, Fediw M, Garcia S, Haragadon A, Jatoi A, Kinczewski LE, Kline-Quiroz C, Loggers ET, Mandrell B, McInnes S, Mooney K, Patel H, Riba MB, Rugo H, Santivasi W, Swetz KM, Venkat P, Wagner-Johnston N, Walter M, Zhou ES. NCCN guidelines version 1.2023 - cancer-related fatigue: National Comprehensive Cancer Network, Inc.; 2022 [updated 12/22/2022]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf.
Ancoli-Israel S, Liu L, Rissling M, Natarajan L, Neikrug AB, Palmer BW, Mills PJ, Parker BA, Sadler GR, Maglione J (2014) Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: a 1-year longitudinal study. Support Care Cancer 22(9):2535–2545
Courtet P, Olié E (2012) Circadian dimension and severity of depression. Eur Neuropsychopharmacol 22(Suppl 3):S476–S481
Wirz-Justice A (2008) Diurnal variation of depressive symptoms. Dialogues Clin Neurosci 10(3):337–343
Germain A, Kupfer DJ (2008) Circadian rhythm disturbances in depression. Hum Psychopharmacol 23(7):571–585
de Rooij BH, Ramsey I, Clouth FJ, Corsini N, Heyworth JS, Lynch BM, Vallance JK, Boyle T (2022) The association of circadian parameters and the clustering of fatigue, depression, and sleep problems in breast cancer survivors: a latent class analysis. J Cancer Surviv. https://doi.org/10.1007/s11764-022-01189-w
Stiglic G, Kocbek P, Fijacko N, Zitnik M, Verbert K, Cilar L (2020) Interpretability of machine learning-based prediction models in healthcare. WIREs Data Mining and Knowledge Discov 10(5):e1379
Rudin C, Chen C, Chen Z, Huang H, Semenova L, Zhong C (2022) Interpretable machine learning: fundamental principles and 10 grand challenges. Stat Surveys 16:1–85
Kaur I, Doja MN, Ahmad T (2022) Data mining and machine learning in cancer survival research: an overview and future recommendations. J Biomed Inform 128:104026
Shehab M, Abualigah L, Shambour Q, Abu-Hashem MA, Shambour MKY, Alsalibi AI, Gandomi AH (2022) Machine learning in medical applications: a review of state-of-the-art methods. Comput Biol Med 145:105458
Srivastava R (2022) Applications of artificial intelligence multiomics in precision oncology. J Cancer Res Clin Oncol 149(1):503–10
Tufail AB, Ma YK, Kaabar MKA, Martínez F, Junejo AR, Ullah I, Khan R (2021) Deep learning in cancer diagnosis and prognosis prediction: a minireview on challenges, recent trends, and future directions. Comput Math Methods Med 2021:9025470
Funding
This work was supported by the National Cancer Institute at the National Institute of Health under grant CA134900, grant CA233774, and grant CA082103. Dr. Olshen is partially supported by the Cancer Center Support Grant (P30CA082103). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Dr. Miaskowski is an American Cancer Society Clinical Research Professor.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data analysis was performed by Kord Kober, Ritu Roy, and Adam Olshen. The first draft of the manuscript was written by Kord Kober and Chris Miaskowski, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Committee on Human Research at the University of California.
Consent to participate
This study was exempted from written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kober, K.M., Roy, R., Conley, Y. et al. Prediction of morning fatigue severity in outpatients receiving chemotherapy: less may still be more. Support Care Cancer 31, 253 (2023). https://doi.org/10.1007/s00520-023-07723-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-07723-5