Abstract
Background
Dehydroepiandrosterone (DHEA) is helpful for treating vaginal symptoms. This secondary analysis evaluated the impact of vaginal DHEA on hormone concentrations, bone turnover, and vaginal cytology in women with a cancer history.
Methods
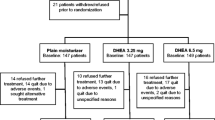
Postmenopausal women, diagnosed with breast or gynecologic cancer, were eligible if they reported at least moderate vaginal symptoms. Participants could be on tamoxifen or aromatase inhibitors (AIs). Women were randomized to 3.25 versus 6.5 mg/day of DHEA versus a plain moisturizer (PM) control. Sex steroid hormone levels, biomarkers of bone formation, vaginal pH, and maturation index were collected at baseline and 12 weeks. Analysis included independent t tests and Wilcoxon rank tests, comparing each DHEA arm with the control.
Results
Three hundred forty-five women contributed evaluable blood and 46 contributed evaluable cytology and pH values. Circulating DHEA-S and testosterone levels were significantly increased in those on vaginal DHEA in a dose-dependent manner compared to PM. Estradiol was significantly increased in those on 6.5 mg/day DHEA but not in those on 3.25 mg/day DHEA (p < 0.05 and p = 0.05, respectively), and not in those on AIs. Biomarkers of bone formation were unchanged in all arms. Maturation of vaginal cells was 100% (3.25 mg/day), 86% (6.5 mg/day), and 64% (PM); pH decreased more in DHEA arms.
Conclusion
DHEA resulted in increased hormone concentrations, though still in the lowest half or quartile of the postmenopausal range, and provided more favorable effects on vaginal cytology, compared to PM. Estrogen concentrations in women on AIs were not changed. Further research on the benefit of vaginal DHEA is warranted in hormone-dependent cancers.

Similar content being viewed by others
References
Gracia CR, Freeman EW (2004) Acute consequences of the menopausal transition: the rise of common menopausal symptoms. Endocrinol Metab Clin N Am 33(4):675–689. https://doi.org/10.1016/j.ecl.2004.07.003
Bacon JL (2017) The menopausal transition. Obstet Gynecol Clin N Am 44(2):285–296. https://doi.org/10.1016/j.ogc.2017.02.008
Ganz PA, Cecchini RS, Julian TB et al (2016) Patient-reported outcomes with anastrozole versus tamoxifen for postmenopausal patients with ductal carcinoma in situ treated with lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet 387(10021):857–865
Cella D, Fallowfield LJ (2008) Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 107(2):167–180. https://doi.org/10.1007/s10549-007-9548-1
Barton DL, Ganz PA (2015) Symptoms: menopause, infertility and sexual health. Adv Exp Med Biol 862:115–141. https://doi.org/10.1007/978-3-319-16366-6_9
Lara LA, Useche B, Ferriani RA et al (2009) The effects of hypoestrogenism on the vaginal wall: interference with the normal sexual response. J Sex Med 6(1):30–39. https://doi.org/10.1111/j.1743-6109.2008.01052.x
Leclair DM, Ananjarajah G (2002) Review article: Effects of estrogen deprivation: vasomotor symptoms, urogenital atrophy, and psychobiologic effects. Clinics in Fam Pract 4(1):27–39. https://doi.org/10.1016/S1522-5720(03)00049-7
Archer DF (2010) Efficacy and tolerability of local estrogen therapy for urogenital atrophy. Menopause 17(1):194–203
Tan O, Bradshaw K, Carr BR (2012) Management of vulvovaginal atrophy-related sexual dysfunction in postmenopausal women: an up-to-date review. Menopause 19(1):109–117
North American Menopause Society (2007) The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause (New York) 14(3 Pt 1):355–369 quiz 370–351
Santen RJ, Pinkerton JV, Conaway M et al (2002) Treatment of urogenital atrophy with low-dose estradiol: preliminary results. Menopause 9(3):179–187
Kendall A, Dowsett M, Folkerd E, Smith I (2006) Caution: vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitors. Ann Oncol 17(4):584–587. https://doi.org/10.1093/annonc/mdj127
Wills S, Ravipati A, Venuturumilli P, Kresge C, Folkerd E, Dowsett M, Hayes DF, Decker DA (2012) Effects of vaginal estrogens on serum estradiol levels in postmenopausal breast cancer survivors and women at risk of breast cancer taking an aromatase inhibitor or a selective estrogen receptor modulator. J Oncol Pract 8(3):144–148. https://doi.org/10.1200/JOP.2011.000352
Naessen T, Rodriguez-Macias K, Lithell H (2001) Serum lipid profile improved by ultra-low doses of 17 beta-estradiol in elderly women. J Clin Endocrinol Metab 86(6):2757–2762. https://doi.org/10.1210/jcem.86.6.7524
Naessen T, Berglund L, Ulmsten U (1997) Bone loss in elderly women prevented by ultralow doses of parenteral 17beta-estradiol. Am J Obstet Gynecol 177(1):115–119. https://doi.org/10.1016/S0002-9378(97)70448-4
Reeder-Hayes K, Muss HB (2017) Vaginal estrogens and aromatase inhibitors: how safe is safe enough? JAMA Oncol 3(3):305–306. https://doi.org/10.1001/jamaoncol.2016.3934
Labrie F, Archer D, Bouchard C et al (2009) Effect of intravaginal dehydroepiandrosterone (prasterone) on libido and sexual dysfunction in postmenopausal women. Menopause 16(5):923–931
Labrie F, Archer D, Bouchard C et al (2009) Intravaginal dehydroepiandrosterone (prasterone), a physiological and highly efficient treatment of vaginal atrophy. Menopause 16(5):907–922
Labrie F, Derogatis L, Archer DF, Koltun W, Vachon A, Young D, Frenette L, Portman D, Montesino M, Côté I, Parent J, Lavoie L, Beauregard A, Martel C, Vaillancourt M, Balser J, Moyneur É, Members of the VVA Prasterone Research Group (2015) Effect of intravaginal prasterone on sexual dysfunction in postmenopausal women with vulvovaginal atrophy. J Sex Med 12(12):2401–2412. https://doi.org/10.1111/jsm.13045
Labrie F, Martel C, Berube R et al (2013) Intravaginal prasterone (DHEA) provides local action without clinically significant changes in serum concentrations of estrogens or androgens. J Steroid Biochem Mol Biol 138:359–367. https://doi.org/10.1016/j.jsbmb.2013.08.002
Samaras N, Samaras D, Frangos E, Forster A, Philippe JA (2013) Review of age-related dehydroepiandrosterone decline and its association with well-known geriatric syndromes: is treatment beneficial? Rejuvenation Res 16(4):285–294. https://doi.org/10.1089/rej.2013.1425
Hlaing TT, Compston JE (2014) Biochemical markers of bone turnover—uses and limitations. Ann Clin Biochem 51(2):189–202. https://doi.org/10.1177/0004563213515190
Peris P, Alvarez L, Monegal A et al (1999) Biochemical markers of bone turnover after surgical menopause and hormone replacement therapy. Bone 2(3):349–353
Tuntiviriyapun P, Panyakhamlerd K, Triratanachat S, Chatsuwan T, Chaikittisilpa S, Jaisamrarn U, Taechakraichana N (2015) Newly developed vaginal atrophy symptoms II and vaginal pH: a better correlation in vaginal atrophy? Climacteric 18(2):246–251. https://doi.org/10.3109/13697137.2014.981520
Weber MA, Limpens J, Roovers JPWR (2015) Assessment of vaginal atrophy: a review. Int Urogynecol J 26(1):15–28. https://doi.org/10.1007/s00192-014-2464-0
Labrie F, Archer D, Bouchard C et al (2009) Serum steroid levels during 12-week intravaginal dehydroepiandrosterone administration. Menopause 16(5):897–906
Barton DL, Sloan JA, Shuster LT, et al Impact of vaginal dehydroepiandosterone (DHEA) on vaginal symptoms in female cancer survivors: Trial N10C1 (Alliance), J Clin Oncol, 2014 ASCO Annual Meeting Abstracts. Vol 32 No 15_suppl (May 20 Supplement), 2014: 9507
Christiansen C, Christensen MS, Larsen NE, Transbol IB (1982) Pathophysiological mechanisms of estrogen effect on bone metabolism. Dose-response relationships in early postmenopausal women. J Clin Endocrinol Metab 55(6):1124–1130. https://doi.org/10.1210/jcem-55-6-1124
Birrell SN, Butler LM, Harris JM, Buchanan G, Tilley WD (2007) Disruption of androgen receptor signaling by synthetic progestins may increase risk of developing breast cancer. FASEB J 21(10):2285–2293. https://doi.org/10.1096/fj.06-7518com
Traish AM, Fetten K, Miner M, Hansen ML, Guay A (2010) Testosterone and risk of breast cancer: appraisal of existing evidence. Horm Mol Biol Clin Invest 2(1):177–190
Hu DG, Selth LA, Tarulli GA et al (2016) Androgen and estrogen receptors in breast cancer coregulate human UDP-glucuronosyltransferases 2B15 and 2B17. Cancer Res 76(19):5881–5893
Labrie F, Luu-The V, Labrie C et al (2003) Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev 24(2):152–182. https://doi.org/10.1210/er.2001-0031
Funding
This work was supported by the National Cancer Institute of the National Institutes of Health (grant UG1CA189823, to the Alliance for Clinical Trials in Oncology NCORP Grant) and also in part by the Public Health Service (grants U10CA025224, U10CA035090, U10CA035101, U10CA035103, U10CA035113, U10CA035119, U10CA035267, U10CA035269, U10CA035415, U10CA035431, U10CA035448, U10CA037404, U10CA037417, U10CA052352, U10CA063848, U10CA063849, U10CA180790, UG1CA189863, and UG1CA189971). This work was also supported in part by funds from a grant from the Breast Cancer Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was supported by the National Cancer Institute and the Breast Cancer Research Foundation. None of the authors have any conflicts of interest with either of these two funding agencies. The work is solely that of the authors and does not reflect the views of the funding agencies. The data is under the control of the Statistics and Data Management group of the Alliance and can be produced in support of the analysis described in this paper, if required.
Rights and permissions
About this article
Cite this article
Barton, D.L., Shuster, L.T., Dockter, T. et al. Systemic and local effects of vaginal dehydroepiandrosterone (DHEA): NCCTG N10C1 (Alliance). Support Care Cancer 26, 1335–1343 (2018). https://doi.org/10.1007/s00520-017-3960-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-017-3960-9