Abstract
Background
Data from US Oncology Adjuvant Trial 9735 has shown that four cycles of docetaxel plus cyclophosphamide (TC) improved disease-free and overall survival when compared against doxorubicin and cyclophosphamide (AC) in early-stage breast cancer. The febrile neutropenia (FN) rate was 4% in this study without primary granulocyte colony-stimulating factors (G-CSF) prophylaxis. However, the incidence of docetaxel-induced myelosuppression is recognized to be higher among Asian population. Hence, this study was designed to evaluate the impact of G-CSF to reduce FN-related events in Asian cancer patients treated with TC.
Method
This retrospective cohort study was conducted on Asian breast cancer patients who have received intravenous docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2 between 2006 to 2008. Patients did not receive oral antibiotic prophylaxis, and prophylactic G-CSF after chemotherapy was prescribed under the discretion of the primary oncologist.
Results
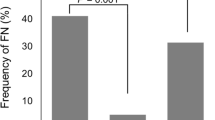
During cycle 1 of chemotherapy, 6.3% patients received G-CSF manifested FN, while 25% patients who did not receive G-CSF manifested FN (RR = 0.252, 95% CI 0.102 to 0.622). Introduction of G-CSF as primary prophylaxis provided an absolute risk reduction of FN events by 18.7%. Chemotherapy doses were maintained throughout all cycles. Patients with pretreatment white blood cell counts (WBC) below 6.0 × 103/mm3 and absolute neutrophil counts (ANC) below 3.1 × 103/mm3 were associated with higher rates of FN during Cycle 1 (p = 0.009, p = 0.007).
Conclusions
Our findings indicate that TC was associated with higher rates of FN than reported in the clinical trial. The 25% incidence fulfills the requirement of primary prophylaxis with G-CSF. Routine administration of G-CSF is highly recommended to reduce the rates of FN in breast cancer patients receiving TC.
Similar content being viewed by others
References
Iannucci A, Chan A Management and treatment of hematologic toxicities. In: Ignoffo RJ, Viele C, Ngo Z (eds) Oncology nursing-pharmacy handbook, 1st edn. Elsevier-Mosby
Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106:2258–2266
Smith TJ, Khatcheressian J, Lyman GH et al (2006) Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24:3187–3205
Lyman GH (2005) Guidelines of the National Comprehensive Cancer Network on the use of myeloid growth factors with cancer chemotherapy: a review of the evidence. J Natl Compr Canc Netw 3:557–571
Kuderer NM, Dale DC, Crawford J, Lyman GH (2007) Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol 25:3158–3167
Sung L, Nathan PC, Alibhai SMH, Tomlinson GA, Beyene J (2007) Meta-analysis: effect of prophylactic hematopoietic colony-stimulating factors on mortality and outcomes of infection. Ann Intern Med 147:400–411
Lyman GH, Kuderer NM, Djulbegovic B (2002) Prophylactic granulocyte colony-stimulating factor in patients receiving dose-intensive cancer chemotherapy: a meta-analysis. Am J Med 112:406–411
Aapro MS, Cameron DA, Pettengell R et al (2006) EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer 42:2433–2453
Jones SE, Savin MA, Holmes FA et al (2006) Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol 24:5381–5387
Jones S, Holmes FA, O'Shaughnessy J et al (2009) Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol 27(8):1177–1183
Hor SY, Lee SC, Wong CI et al (2008) PXR, CAR and HNF4? genotypes and their association with pharmacokinetics and pharmacodynamics of docetaxel and doxorubicin in Asian patients. Pharmacogenomics 8:139–146
Segal BH, Freifeld AG, Baden LR et al (2008) Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw 6:122–174
Jenkins P, Freeman S (2009) Pretreatment haematological laboratory values predict for excessive myelosuppression in patients receiving adjuvant FEC chemotherapy for breast cancer. Ann Oncol 20(1):34–40
Zou G (2004) A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 159(7):702–706
Cheung YB (2007) A modified least-squares regression approach to the estimation of risk difference. Am J Epidemiol 166(11):1337–1344
Soong D, Haj R, Leung MG et al (2009) High rate of febrile neutropenia in patients with operable breast cancer receiving docetaxel and cyclophosphamide. J Clin Oncol 27(26):e101–e102
Ferguson T, Wilcken N, Vagg R, Ghersi D, Nowak A (2007) Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst Rev
Verschraegen CF, Sittisomwong T, Kudelka AP et al (2000) Docetaxel for patients with paclitaxel-resistant Mullerian carcinoma. J Clin Oncol 18:2733–2739
Martin M, Lluch A, Segui MA et al (2006) Toxicity and health-related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and cyclophosphamide (FAC): impact of adding primary prophylactic granulocyte-colony stimulating factor to the TAC regimen. Ann Oncol 17:1205–1212
Vogel CL, Wojtukiewicz MZ, Carroll RR et al (2005) First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol 23:1178–1184
Nabholtz JM, Falkson C, Campos D et al (2003) Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first-line chemotherapy for metastatic breast cancer: results of a randomized, multicenter, phase III trial. J Clin Oncol 21:968–975
Van Cutsem E, Moiseyenko VM, Tjulandin S et al (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V25 study group. J Clin Oncol 24:4991–4997
Jones SE, Erban J, Overmoyer B et al (2005) Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol 23:5542–5551
Goh BC, Lee SC, Wang LZ et al (2002) Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. J Clin Oncol 20:3683–3690
Alexandre J, Rey E, Girre V et al (2007) Relationship between cytochrome 3A activity, inflammatory status and the risk of docetaxel-induced febrile neutropenia: a prospective study. Ann Oncol 18:168–172
Tsai SM, Lin CY, Wu SH et al (2009) Side effects after docetaxel treatment in Taiwanese breast cancer patients with CYP3A4, CYP3A5, and ABCB1 gene polymorphisms. Clin Chim Acta. doi:10.1016/j.cca.2009.03.038
Hughes WT, Armstrong D, Bodey GP et al (2002) Guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 34:730–751
Papaldo P, Lopez M, Marolla P et al (2005) Impact of five prophylactic filgrastim schedules on hematologic toxicity in early breast cancer patients treated with epirubicin and cyclophosphamide. J Clin Oncol 23:6908–6918
Wilson-Royalty M, Lawless G, Palmer C, Brown R (2001) Predictors for chemotherapy-related severe or febrile neutropenia: a review of the clinical literature. J Oncol Pharm Pract 7:141–147
Acknowledgments
Authors would like to acknowledge the Department of Pharmacy, National University of Singapore for providing Final Year Project funding for this project.
Conflict of interest
The authors have no conflicts of interest that are directly relevant to the content of this study.
Authors’ contributions
AC, VS and RN are involved with the planning of the project, writing the manuscript, performing data analysis and interpretation of data. FWH and CJC are involved with the data collection and data analysis of the study. TSH has performed the statistical analysis of the study. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chan, A., Fu, W.H., Shih, V. et al. Impact of colony-stimulating factors to reduce febrile neutropenic events in breast cancer patients receiving docetaxel plus cyclophosphamide chemotherapy. Support Care Cancer 19, 497–504 (2011). https://doi.org/10.1007/s00520-010-0843-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-010-0843-8