Abstract
Goals of the work
To assess the relationship between oral mucositis (OM) and adverse clinical and economic outcomes in patients with hematologic malignancies receiving allogeneic hematopoietic stem-cell transplantation (HSCT).
Materials and methods
A retrospective chart review study of 281 allogeneic HSCT recipients with hematologic malignancies was undertaken at a single academic center. OM extent and severity were assessed across eight oropharyngeal sites using a validated scale, which was scored as follows: no erythema/ulceration=0; erythema only=I; ulceration, one site=II; ulceration, two sites=III; ulceration, three sites=IV and ulceration, four or more sites=V. OM assessments began on the day of conditioning and continued twice weekly within 28 days or hospital discharge. Analyses examined the relationship between the worst OM grade and selected adverse outcomes, including days with fever, days of total parenteral nutrition (TPN), days of parenteral narcotic therapy, incidence of significant (common terminology criteria (CTC) grade 3 or 4) infection, mortality and inpatient days and charges.
Main results
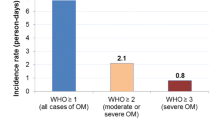
The mean age of the study subjects was 41 years. Of the patients, 96% (n = 269) received total body irradiation and 76% (n = 214) experienced an OM grade of ≥II (i.e., ulceration). The worst OM grade was significantly (p < 0.05) associated with the number of days of TPN and parenteral narcotic therapy, number of days with fever, incidence of significant infection, time in hospital and total inpatient charges.
Conclusions
OM is associated with worse clinical and economic outcomes in patients with hematologic malignancies undergoing allogeneic HSCT.


Similar content being viewed by others
References
Bellm LA, Epstein JB, Rose-Ped A et al (2000) Patient reports of complications of bone marrow transplantation. Support Care Cancer 8:33–39
Cutler C, Li S, Kim HT et al (2005) Mucositis after allogeneic hematopoietic stem cell transplantation: a cohort study of methotrexate- and non-methotrexate-containing graft-versus-host disease prophylaxis regimens. Biol Blood Marrow Transplant 11(5):383–388
Elting LS, Cooksley C, Chambers M et al (2003) The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer 98:1531–1539
Hwang WYK, Koh L-P, Ng HJ et al (2004) A randomized trial of amifostine as a cytoprotectant for patients receiving myeloablative therapy for allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 34:51–56
McGuire DB, Altomonte V, Peterson DE et al (1993) Patterns of mucositis and pain in patients receiving preparatory and bone marrow transplantation. Oncol Nurs Forum 20:1493–1502
Monopoli M, Woo S, Valley M, Sonis S (1991) A site-based scoring system of chemotherapy-induced oral mucositis in bone marrow transplant recipients. Proceedings of the Annual Meeting of the Society for Oral Oncology (abstract)
Rapoport AP, Miller Watelet LF, Linder T et al (1999) Analysis of factors that correlate with mucositis in recipients of autologous and allogeneic stem-cell transplants. J Clin Oncol 17:2446–2453
Robien K, Schubert MM, Bruemmer B et al (2004) Predictors of oral mucositis in patients receiving hematopoietic cell transplants for chronic myelogenous leukemia. J Clin Oncol 22:1268–1275
Ruescher TJ, Sodeifi A, Scrivani SJ et al (1998) The impact of mucositis on alpha-hemolytic streptococcal infection in patients undergoing autologous bone marrow transplantation for hematologic malignancies. Cancer 82(11):2275–2281
Sonis ST, Oster G, Fuchs H et al (2001) Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol 19(8):2201–2205
Spielberger R, Stiff P, Bensinger W et al (2004) Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med 351:2590–2598
Wardley AM, Jayson GC, Swindell R et al (2000) Prospective evaluation of oral mucositis in patients receiving myeloablative conditioning regimens and haemopoeitic progenitor rescue. Br J Haematol 110:292–299
Woo SB, Sonis ST, Monopoli MM et al (1993) A longitudinal study of oral ulcerative mucositis in bone marrow transplant recipients. Cancer 72:1612–1617
Zerbe MB, Parkerson SG, Ortlieb ML et al (1992) Relationships between oral mucositis and treatment variables in bone marrow transplant patients. Cancer Nurs 15:196–205
Acknowledgement
Financial support was provided by Amgen, Thousand Oaks, CA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vera-Llonch, M., Oster, G., Ford, C.M. et al. Oral mucositis and outcomes of allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies. Support Care Cancer 15, 491–496 (2007). https://doi.org/10.1007/s00520-006-0176-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-006-0176-9