Abstract
Aims
To assess incidence, predictability, preventability and severity of adverse drug reactions (ADRs) in hospitalised oncology patients.
Patients and methods
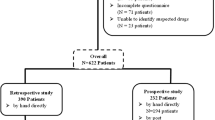
Patients hospitalised at Peter MacCallum Cancer Centre from 28 February to 2 June 2000 were selected for interviews about symptoms related to their drug therapy. Medical records were also reviewed. Causality, predictability, preventability and severity were assessed for each ADR.
Results
One hundred and sixty-seven patients associated with 171 admissions were interviewed. Four hundred and fifty-four ADRs were identified in 127 (74.3%) separate admissions (mean ADRs per admission 2.7; range 0–18). Eighty-eight percent of ADRs were predictable. Of these, 1.6% was classified as definitely preventable and 46.1% probably preventable. The ten most common ADRs were constipation, nausea ± vomiting, fatigue, alopecia, drowsiness, myelosuppression, skin reactions, anorexia, mucositis and diarrhoea. These ADRs have high-documented incidence rates and were also the ten most predictable ADRs in this study. Common reasons for ADRs to be assessed as definitely or probably preventable were omission or inadequate/inappropriate use of preventative measures. The results also showed a discrepancy between clinical severity and patients’ perception of the impact of ADRs on well being.
Conclusions
ADRs are common in hospitalised oncology patients and are predictable and at least probably preventable in many instances. Improved use of preventative measures has the potential to contribute to reducing the incidence and severity of ADRs. Recognition and understanding of the discrepancy that exists between clinical severity and patient-perceived severity of ADRs will enable specific areas to be identified and targeted for vigourous intervention.




Similar content being viewed by others
References
American Hospital Formulary Service drug information (1984) Board of Directors of the American Society of Hospital Pharmacists, Bethesda
Audit Commission (2001) A spoonful of sugar—medicines management in NHS hospitals. Audit Commission, London
Australian adverse drug reactions bulletin (1998) Australian Adverse Drug Reaction Advisory Committee (ADRAC)
Australian prescription products guide (2000) 29th edn. Australian Pharmaceutical Publishing
Bates D, Leape L, Petrycki S (1993) Incidence and preventability of adverse drug events in hospitalized adults. J Gen Intern Med 8:289–294
Council for International Organisation for Medical Science (CIOMS) (1994) WGI, Guidelines for preparing core clinical-safety information on drugs. Council for International Organisation for Medical Sciences, Geneva
Clinical Oncological Society of Australia (2002) Optimising cancer care in Australia. The Cancer Council Australia and the National Cancer Council Initiative, Melbourne, pp 1–122
Coates AS, Abraham SB, Kaye T et al (1983) On the receiving end—patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol 19:203–208
Dewland P, Dewland J (1999) At the coalface, but on the receiving end. J Med Ethics 25:541–546
Dormann H, Muth-Selbach U, Krebs S et al (2000) Incidence and costs of adverse drug reactions during hospitalisation: computerised monitoring versus stimulated spontaneous reporting. Drug safety 22:161–168
Dukes J, Chalker M, Leuwer PKM et al (2000) Meyler’s side effects of drugs, 14th edn. Elsevier, New York
Fisher S, Kent TA, Bryant SG (1995) Postmarketing surveillance by patient self-monitoring: preliminary data for sertraline versus fluoxetine. J Clini Psych 56:288–296
Foddy W (1994) Constructing questions for interviews and questionnaires. Theory and practice in social research. Cambridge University Press
Green CF, Mottram DR, Rowe PH, Pirmohamed M (2000) Adverse drug reactions as a cause of admission to an acute medical assessment unit: a pilot study. J Clin Pharm Ther 25:355–361
Griffin AM, Butow PN Coates AS et al (1996) On the receiving end. V: Patient perceptions of the side effects of cancer chemotherapy in 1993. An Oncol 7:189–195
Jarernsiripornkul N, Krska J, Capps PA et al (2002) Patient reporting of potential adverse drug reactions: a methodological study. Br J Clin Pharmacol 53:318–325
Mjorndal T, Boman MD, Hagg S et al (2002) Adverse drug reactions as a cause for admissions to a department of internal medicine. Pharmacoepidemiol Drug Saf 11:65–72
Naranjo C, Busto U, Sellars E et al(1981) A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30:239–245
National hospital morbidity data collection—Australia (excluding NT) (1992–1993)
Pon D (1996) Service plans and clinical interventions targeted by the oncology pharmacist. Pharm Pract Manag 16:18–30
PREMEDLINE and MEDLINE version: rel 6.1.0, source ID 1.7672.1.63
Roughead EE, Gilbert AL, Primrose JG, Sansom LN (1998) Drug-related hospital admissions: a review of Australian studies published 1988–1996. Medi J Aust 168:405–408
Schumock G, Thornton J (1992) Focusing on the preventability of adverse drug reactions. Hosp Pharm 27:538
Tegeder I, Levy M, Muth-Selbach U et al (1999) Retrospective analysis of the frequency and recognition of adverse drug reactions by means of automatically recorded laboratory signals. Br J Clin Pharmacol 47:557–564
Thomson MICROMEDEX. MICROMEDEX(R) Health-care Series Vol. 117. http://micromedex.hcn.net.au/mdx-full/. Cited 19 Oct 2003
White TJ, Arakelian A, Rho JP (1999) Counting the costs of drug-related adverse events. Pharmacoeconomics 15:445–458
Wilson R, Runciman W, Gibberd R et al (1995) Quality in Australian health care study. Med J Aust 163:458–471
World Health Organization (1999) Common toxicity criteria (CTC). 1 August 1999
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lau, P.M., Stewart, K. & Dooley, M. The ten most common adverse drug reactions (ADRs) in oncology patients: do they matter to you?. Support Care Cancer 12, 626–633 (2004). https://doi.org/10.1007/s00520-004-0622-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-004-0622-5