Summary
Objective
To increase knowledge of discrete symptoms shall help to avoid misinterpretation of test results and to gain better understanding of associations between early symptoms and severe disease to provide additional criteria for targeted early interventions.
Design
Retrospective observational study.
Setting
Austrian GP practices in the year 2020, patients above 18 years were included.
Participants
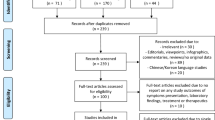
We recruited 25 practices which included 295 participants with a positive SARS-CoV‑2 test.
Main outcome measures
Data collection comprised basic demographic data, risk factors and the recording of symptoms at several points in time in the course of the illness. Descriptive analyses for possible associations between demographics and symptoms were conducted by means of cross tabulation. Group differences (hospitalized yes/no) were assessed using Fisher’s exact test. The significance level was set to 0.05; due to the observational character of the study, no adjustment for multiplicity was performed.
Results
Only one third of patients report symptoms generally understood to be typical for COVID‑19. Most patients presented with unspecific complaints. We found symptoms indicating complicated disease, depending on when they appear. The number of symptoms may be a predictor for the need of hospital care. More than 50% of patients still experience symptoms 14 days after onset.
Conclusion
Unspecific symptoms are valuable indicators in the detection of early COVID‑19 disease that practitioners and the general public should be aware of also in the interpretation of low sensitivity tests. Monitoring patients using the indicators we identified may help to identify patients who are likely to profit from early intervention.
Similar content being viewed by others
Introduction
A central aspect in the containment of the coronavirus disease 2019 (COVID-19) pandemic is identification and isolation of possibly infectious persons, to prevent further spreading of the disease. Several studies were conducted with the goal of identifying diagnostic criteria that enable clinical differentiation between COVID-19 and non-COVID-19 infections: most investigations used data collected from hospitalized patients [1,2,3,4,5,6], i.e. from patients with severe disease. These studies have found high prevalence of fever (around 90%), dyspnea (up to 50%), cough (60–70%), and fatigue in patients with COVID-19. Several other studies evaluated self-reported data from symptom tracker apps or outpatient clinics [2, 7,8,9,10]. We could find only one investigation including additional data derived from primary care health records [9]. Studies conducted in non-hospitalized patients reported a lower prevalence of the symptoms mentioned above and a wide spectrum of additional symptoms, such as myalgia, rhinorrhea and/or nasal congestion, headache, sore throat, gastrointestinal and cardiovascular disturbances [7,8,9, 11]. Loss of taste and/or smell was found to be specific when present [8, 11]. All patients included in those studies had gone through a selection process before testing, by case definitions and testing criteria, by epidemiological factors or by previous investigations, such as computed tomography (CT) scans of the lungs. Some of these studies included only patients who had tested positive [10], others investigated patients who had tested positive or negative [8, 9]. Patients not fulfilling established criteria may have escaped testing, and the symptoms found may reflect testing criteria [7]. Some authors suspected that possibly a large proportion of COVID-19 cases are never tested and, thus, never recorded [12]. This has not been investigated so far.
What this study can add
Patients presenting with other than the canonical symptoms might be overlooked by current testing strategies and screening tools. Patients with discrete and seemingly unsuspicious complaints tend to be mobile and can widely spread the disease. Awareness among stake holders as well as in the general public as to the wide range of uncharacteristic symptoms is most needed to promote low-threshold and high sensitivity testing, and to advise repeated testing if any symptoms are present, including unspecific, non-respiratory ones. This seems a requirement for effective containment strategies.
Austrian general practitioners (GPs) are entitled to make an individual testing decision according to clinical judgement like when there is no alternative explanation for the symptom presented. Austrian GP practices can send their own samples for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) PCR to be analyzed via a surveillance network based at the Medical University Vienna, and from mid-October 2020 point of care testing for SARS-CoV‑2 in GP’s offices has become possible, thus 25% of our study practices were part of this network. GP practices in Austria are easy to access; in general, it is possible to walk in or to get an appointment on the same day.
Against this background, it was the aim of this study to assess early COVID-19 symptoms and their development in patients of different demographic groups in primary care as well as their possible associations with complications in the course of the disease.
Patients, material and methods
This study was designed as an observational study in general practice in Austria. Recruitment of practices and participants took place between July 2020 and December 2020, thus comprising infections with the SARS-CoV‑2 wild type, which was the only one circulating in Austria at that time.
The Austrian Society of General Practice and Family Medicine invited their members, publicly funded GPs and their practices, to participate. For this purpose, first announcements and invitation letters were sent out between April and July 2020. After receiving a positive vote by the Ethics Committee of the Karl-Landsteiner University for Health Sciences, practices interested in participating were informed about the aims of this study in detail and after agreement study material was provided.
Participating GPs included patients above 18 years either after testing positive at the point of care or after reporting to their GP with a positive PCR test from another testing facility. These persons were invited to participate in this study. If they were willing to participate and after the provision of written informed consent, they were included in the study.
Study material and data collection
A questionnaire using the open-source CDC program Epi Info 7 [13] was designed to record demographic and anamnestic data, comorbidities, medication groups and risk factors regarding COVID-19 (supplementary material 1). Data extracted from the electronic health records (EHR) of the practices were transferred to the questionnaire. Clinical parameters for assessing the patients’ health status over a period of 2 weeks starting with the day of symptom onset as day 1, were documented. Further assessment days were days 5, 7, 8, 10 and 14. Clinical data were self-reported in a monitoring sheet either by the patients themselves or acquired via telephone calls by the GPs. They were transferred to the questionnaire by the GP’s offices. Data regarding patients’ health status and symptoms were temperature >38°, blood pressure, heart rate, dyspnea, chest pressure, tightness of chest, malaise, weakness, headache, rhinitis, anosmia, ageusia, sore throat and gastrointestinal symptoms (supplemental material 2). These clinical parameters were selected using published studies of signs and symptoms of patients with COVID-19 [14, 15]. All data were pseudonymized before forwarding them to the study center where the data were checked for completeness and correct entries. Ambiguities were clarified by telephone call or e‑mail contact with the participating GP practice.
Data analysis
In a first step we analyzed the data concerning demographics and symptoms during the acute phase of COVID-19 disease. These results are presented in this paper. Influence of comorbidities and other risk factors, e.g. patients’ age at hospitalization and long-term complications will be evaluated in a next step.
Data were converted into Excel files and accompanying statistical analysis was done using the statistical software program R (version 3.5.1) [16].
Participants’ demographics as well as symptoms were first analyzed descriptively. Descriptive analyses for possible associations between demographics and symptoms were conducted by means of cross tables. Group differences (hospitalized yes/no) were assessed using Fisher’s exact test. The significance level was set to 0.05; due to the observational character of the study, no adjustment for multiplicity was performed.
Patient involvement
Study design included a protected patient information (PPI) leaflet. Patients were asked if they would like to participate in this study by their GPs, who informed them about the study’s goal and the proceedings involved. They were handed written information and were asked if they had any further questions. After giving written consent they either received a monitoring sheet to record their symptoms and to be returned after completion, or their GP arranged for regular monitoring telephone calls. The monitoring sheet also contained advice on situations requiring immediate medical attention, such as higher degree dyspnea. Patients remained under their physicians’ care during the full duration of the study, since it was the GPs who collected the data to pass them on to the study center after pseudonymization.
The GPs were asked to involve any patient with a SARS-CoV‑2 infection, they were instructed on how to collect patient data and how and why to perform the monitoring. They will be informed about the results of study and their possible consequences for patients’ care via newsletters, podcasts and publications.
Results
Altogether, 25 GP practices and 295 patients from 7 of 9 Austrian federal states could be recruited. On average, the practices recruited 12 patients (SD 8.94, min 2–max 31).
As shown in Table 1 in detail, slightly more women than men were included in the study. In addition, the percentage of obese persons (body mass index [BMI] >30) was 19.0%, which is slightly higher than the general Austrian average of 16% [17]. On the other hand, only 7% of participants were smokers, which is less than half of the Austrian average of 20% [18]. Due to practical reasons smoking status was recorded following the GP’s reporting.
Initial symptoms and development of symptoms
The most common out of the 13 symptoms to be selected were joint or muscle pain and malaise on day 1, each of them reported by half of the patients (Figs. 1 and 2). Loss of smell/taste was reported on day 1 by less than a quarter of patients but became the most frequently expressed complaint from day 7 onwards until the end of the observational period on day 14. Fatigue was the 3rd most prevalent symptom on day 1, and the most common symptom on day 5 and was still highly prevalent on day 10. Therefore, fatigue was found to be the most persistent of symptoms of all. Cough turned out to be a less common symptom on day 1 (5th of 13 symptoms), becoming more frequent from day 5 onwards. Fever >38° was reported by one third of participants as an initial symptom.
Associations between symptoms and hospitalization
Analysis for associations of symptoms with the likelihood of hospitalization yielded the following results: for presence of fever and/or malaise from day 5 onwards we found significant associations with the need for hospitalization sometime in the further course of the disease. Headache showed a significant association if present on day 10. The likelihood for hospitalization was significantly increased in patients with either dyspnea, fatigue, tightness of chest and cough. In contrast, persons with rhinitis, sore throat, chest pain and anosmia as an initial symptom were less likely to need hospital care (Table 2; Fig. 3).
COVID-19 seems to start with several symptoms simultaneously: Nearly half of the participants reported 3–5 symptoms on day 1, 10 participants had not reported symptoms on day 1, with 3 of them having become symptomatic by day 5 and 7 remained asymptomatic (Fig. 4).
Of the patients 68% reported symptoms persisting on day 10, and half the sample still had complaints on day 14.
A higher number of symptoms was associated with higher probability of hospital admission. This was significant for days 7 and 8 (Fig. 3).
Discussion
Initial symptoms and development of symptoms
We found early symptoms to be mostly unspecific and often discreet, with joint or muscle pain, malaise and fatigue being the most common symptoms (Figs. 1 and 2). One of our findings is that none of the 13 symptoms in our selection is either sensitive or specific enough for the early stage of COVID-19 to serve as testing criteria, which is supported by several of the more recent studies based at least partly on data from primary care [7, 9].
Symptoms generally considered to increase the likelihood for COVID-19 diagnosis, i.e. fever, dyspnea and loss of taste and/or smell [7, 9, 10, 19, 20] were found to be comparatively rare, at least at early stages of the disease (Figs. 1 and 2). On day 1 less than 30% of patients reported fever, less than one third (25.9%) reported loss of smell or taste and only 13.6% suffered from dyspnea, which renders those symptoms not well suited as testing criteria. These results differ from other studies that had derived data from hospitalized patients exclusively, or in combination with self-reported data using symptom apps. All of them were conducted in patients who had been tested according to pre-established testing criteria [8, 10]. Our findings suggest that neither presence nor absence of any symptom is suitable to rule out COVID-19, and confirmed results from another study showing that patients reporting any kind of symptoms of a broad range of diseases profit from testing without delay and without restrictions [21].
Fever, cough, and dyspnea have been considered relevant for case definitions and used as screening criteria in many countries for a long time, and to this day are widely understood as characteristic by the general public and mass media. According to our findings this could be an obstacle to correct early diagnosis and case finding. In our sample, none of those symptoms was experienced by more than slightly over a third of the patients at any time during the illness.
This finding may have some impact on testing strategies. Austria like other countries propagates low threshold testing by self-testing kits, which mostly lack external validation of their sensitivity and specificity [22]. Sensitivity varies considerably altogether between the lateral flow tests (LFT) being marketed at the time of the study [23]. We found presence of any of the discreet and unspecific symptoms we investigated to possibly indicate COVID‑19 infection and to increase pre-test probability, thus further reducing sensitivity. Users and decision makers need to be aware of this to avoid misinterpretation of a negative test result [24].
Anosmia is known to be the most specific symptom [8] but according to our results tends to appear in the later course of the disease. As an initial symptom we could trace it in only 22% of patients, but in twice as many on days 7 and 8 (Fig. 1). Menni [8] identified this symptom in 64.5% of patients and concluded that this symptom could help early diagnosis. Our findings do not support this conclusion. The difference may be caused by the time of detection or selection bias and thus shows the relevance of early investigation of symptoms at a low threshold point of care [14].
This finding should lead to reconsider contact-tracing strategies: finding the symptom anosmia might indicate delayed diagnosis and should prompt an extension of the contact tracing period to at least 7 days before the infection was detected.
Associations between symptoms and hospitalization
We found several associations between symptoms and the need for hospitalization. Our findings suggest that dyspnea is prone to lead to admission to hospital when present on day 1, as well as on day 10 and significantly on day 14. Fatigue as well as malaise through the whole course of the disease starting from day 5 and cough persisting on day 8 seem to be associated with higher rates of hospitalization, fever only if present from day 5 on (Table 2; Fig. 3). Dyspnea has been shown to be predictive for hospital admission in one systematic review and a meta-analysis [2, 19], which is in line with our findings. No other predictive symptoms has consistently been identified so far. Our findings on persisting symptoms at the end of the observational period fit in well with recent studies on Long Covid [25, 26].
We observed a higher number of symptoms (3 or more) on days 7 and 8 to be associated with a significantly higher probability of hospital admissions (Fig. 3). Patients in the non-hospitalized group experience a peak in number of symptoms on day 1 to day 5. Patients in the hospitalized group had more symptoms from the start and experienced a further rise on day 5 as well as a markedly slower decrease. This could indicate that patients might profit from being closely monitored: Finding a rise in number of symptoms should arouse suspicion of an imminent severe course of disease. This might, if corroborated, even allow identification of individual cut-off points to introduce innovative early interventions presently under discussion like budesonide, low-molecular heparins or monoclonal antibody therapy [27, 28].
Over 50% of patients reported symptoms at the end of the observational period on day 14, and more than two thirds on day 10, when the isolation period usually ends. Mostly this concerns loss of taste or smell or fatigue. This coincides with the most common complaints reported by patients suffering from Long Covid [25]. Regarding the fact that Long Covid is in many cases not a trivial complaint but may lead to delayed and severe complications [29], it seems justifiable to recommend medical examination after isolation to decide if and when a patient can be considered healthy and safe to return to physical activity and/or work.
Strengths and limitations of this study
This study to our knowledge is the first one to investigate data on the course of COVID-19 collected exclusively from patients in primary care. The GPs were free to make their own testing decision according to their clinical judgement, and they followed each patient individually from day 1 to day 10 or 14 in most cases. Only 10% of the patients included in our study approached primary care after having been tested according to formal testing criteria by another testing facility. Most other studies [2, 7, 9, 10] recruited patients mainly or exclusively via symptom apps or in hospital care, after the testing decision had been made according to established testing criteria, which makes them less likely to detect early symptoms not already known to be associated with COVID-19.
The study has several limitations though. We could recruit 25 practices in 7 out of the 9 Austrian provinces. The average number of patients included per practice was 12 (SD 8.9). The limited number of patients is probably due to the increased workload under difficult working conditions during the pandemic in combination with a rather extensive questionnaire and the need to follow patients over a longer period as well as the effort not being remunerated. We have to reckon with possible recruiting bias; however, the number of cases needed to identify group differences was calculated in advance, and this number has been reached. Our overall results are in accordance with our preliminary result analyses.
Another limitation is that not all data on symptoms were provided by the GPs, particularly on temperature. Most likely this applies mainly for symptoms which were not present but this will have to be clarified by further research. Some patients (approximately 10%) were not diagnosed in primary care, so a possible confounder testing criteria cannot entirely be ruled out. We are likely to have overestimated some symptoms on day 1, because a proportion of patients may have unknowingly been diagnosed later than that.
We could not detect specific patterns of symptom combinations. This may be due to the limited sample size.
Conclusion
We could demonstrate a variety of unspecific symptoms to be clearly more common than those widely understood to be typical of COVID-19. Understanding this can help to avoid missing infectious patients because inappropriate testing methods like testing kits of low sensitivity are being used. This applies particularly since negative antigen tests are accepted for the Green Pass, and new waves of infections with new variants are considered a threat to re-opening strategies after lock-down measures. Negative results in persons with any of the symptoms we identified should be confirmed by PCR testing.
We found several symptoms possibly indicating future complications. This knowledge in conjunction with timely identification in primary care could help to avert severe disease in some cases. To facilitate follow-up in primary care, patients need to be either diagnosed there, or to reliably report to their GP if tested positive.
Implications for further research
Data collected in primary care settings can provide additional information and can offer a wider spectrum of understanding COVID-19 disease. The results of our exploratory retrospective study should be controlled by a prospective study.
We did not record data on patients who tested negative or were not tested at all. Future investigations into this topic should aim at recording symptoms in all patients reporting for suspected SARS-CoV‑2 infection.
References
Guan W, Ni Z, Hu Y, et al. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.
Jain V, Yuan J‑M. Systematic review and meta-analysis of predictive symptoms and comorbidities for severe COVID-19 infection. medRxiv. 2020;2020:2020.03.15.20035360..
Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9.
Fu L, Wang B, Yuan T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J Infect. 2020;80(6):656–65.
Sun P, Qie S, Liu Z, Ren J, Xi J. Clinical characteristics of 50466 patients with 2019-nCoV infection. medRxiv. 2020;2020:2020.02.18.20024539.
Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV‑2 admitted to ICus of the Lombardy region, Italy. JAMA. 2020 Apr 28;323(16):1574–1581.
Struyf T, Deeks JJ, Dinnes J, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19. Cochrane Database Syst Rev. 2021;2:Cd13665.
Menni C, Sudre CH, Steves CJ, Ourselin S, Spector TD. Quantifying additional COVID-19 symptoms will save lives. Lancet. 2020;395(10241):e107–e8.
Mizrahi B, Shilo S, Rossman H, et al. Longitudinal symptom dynamics of COVID-19 infection. Nat Commun. 2020;11(1):6208.
Huang Y, Radenkovic D, Perez K, Nadeau K, Verdin E, Furman D. Age-dependent and independent symptoms and comorbidities predictive of COVID-19 hospitalization. medRxiv. 2020;2020:2020.08.14.20170365.
Menni C, Valdes AM, Freidin MB, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26(7):1037–40.
Alvarez E, Marsico F. COVID-19 mild cases determination from correlating COVID-line calls to reported cases. 2020.
CDC Open Source. Epi info 7.. https://www.cdc.gov/epiinfo/pc.html. Accessed 8 Nov 2021.
Greenhalgh T, Koh GCH, Car J. Covid-19: a remote assessment in primary care. BMJ. 2020;368:m1182.
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2017. https://www.R-project.org/. Accessed 8 Nov 2021.
Statista Austria. BMI. http://www.statistik.at/web_de/statistiken/menschen_und_gesellschaft/gesundheit/gesundheitsdeterminanten/bmi_body_mass_index/025420.html. Accessed 8 Mar 2021.
Statista Austria. Smoking. https://de.statista.com/statistik/daten/studie/510314/umfrage/anteil-der-raucher-gelegenheits-ehemaligen-und-nichtraucher-in-oesterreich/. Accessed 6 Mar 2021.
Case definition Austria. https://www.sozialministerium.at/Themen/Gesundheit/Uebertragbare-Krankheiten/Infektionskrankheiten-A-Z/Neuartiges-Coronavirus.htmlpatients. Accessed 6 Mar 2021.
bmj. Case definition. https://bestpractice.bmj.com/topics/en-gb/3000201/criteria. Accessed 8 Mar 2021.
Leber W, Lammel O, Redlberger-Fritz M, et al. RT-PCR testing to detect a COVID-19 outbreak in Austria: rapid, accurate and early diagnosis in primary care (The REAP study). medRxiv. 2020;2020:2020.07.13.20152439.
Fitzpatrick MC, Pandey A, Wells CR, Sah P, Galvani AP. Buyer beware: inflated claims of sensitivity for rapid COVID-19 tests. Lancet. 2021;397(10268):24–5.
Dinnes J, Deeks JJ, Berhane S, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV‑2 infection. Cochrane Database Syst Rev. 2021 Mar 24;3:CD013705. PMID: 32845525; PMCID: PMC8078202.
Guglielmi G. Rapid coronavirus tests: a guide for the perplexed. Nature. 2021;590(7845):202–5.
Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–31.
Sivan M, Taylor S. NICE guideline on long covid. BMJ. 2020;371:m4938.
Ramakrishnan S, Nicolau DV Jr., Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. The Lancet Respiratory Medicine, Volume 9, Issue 7, 763–772.
Taylor PC, Adams AC, Hufford MM, de la Torre I, Winthrop K, Gottlieb RL. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol. 2021 Jun;21(6):382–393.
Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026.
Acknowledgements
The Austrian Society of General Practice and Family Medicine provided support by assisting the process of recruitment of study practices. This was essential for this study to be undertaken. We thank all our colleague GPs who undertook the task of recruiting patients, completing questionnaires and onward them to us and we thank the many patients, who contributed their data and recorded their symptoms.
Funding
A grant was provided by the Styrian Academy of General Medicine, Graz.
Funding
Open access funding provided by Karl Landsteiner Privatuniversität für Gesundheitswissenschaften
Author information
Authors and Affiliations
Contributions
Rabady S: corresponding author and first author. Hoffman K: equally contributing first author. Brose M: contributing to design of questionnaire and data acquisition and data analysis, revision of manuscript. Lammel O: contributing to design of study, data acquisition, revision of manuscript. Poggenburg S: contributing to design of study, data acquisition, revision of manuscript. Redlberger-Fritz M: contributing to design, evaluation PCR tests, revision of manuscript. Stiasny K: contributing to design, evaluation PCR tests, revision of manuscript. Wendler M: contributing to design of study, data acquisition, revision of manuscript. Weseslindtner L: contributing to design, evaluation PCR tests, revision of manuscript. Zehetmayer S: Contributing to study design, planning of methods, data analysis. Kamenski G: Contributing to design of study, design of questionnaire, data acquisition and data analysis, revision of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
S. Rabady, K. Hoffmann, M. Brose, O. Lammel, S. Poggenburg, M. Redlberger-Fritz, K. Stiasny, M. Wendler, L. Weseslindtner, S. Zehetmayer and G. Kamenski declare that they have no competing interests.
Ethical standards
Ethical approval was given by Commission for Scientific Integrity and Ethics, Karl-Landsteiner Private University for Health Sciences, Krems, Austria. EK Nr: 1046/2020 13/07/20. The study has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The study was registered as DRKS00022448 on DRKS. Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors S. Rabady and K. Hoffmann contributed equally to the manuscript.
Data availability statement
All data referred to in the manuscript are available from: Department of General Medicine and Family Practice, Karl Landsteiner Privatuniversitaet, Krems, Austria
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rabady, S., Hoffmann, K., Brose, M. et al. Symptoms and risk factors for hospitalization of COVID-19 presented in primary care. Wien Klin Wochenschr 134, 335–343 (2022). https://doi.org/10.1007/s00508-021-01992-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-021-01992-y