Abstract
The main objectives of this study were to (i) assess variation within fine particles (PM2.5) and tropospheric ozone (O3) time series in Khorramabad (Iran) between 2019 (before) and 2020 (during COVID-19 pandemic); (ii) assess relationship between PM2.5 and O3, the PM2.5/O3 ratio, and energy consumption; and (iii) estimate the health effects of exposure to ambient PM2.5 and O3. From hourly PM2.5 and O3 concentrations, we applied both linear–log and integrated exposure–response functions, city-specific relative risk, and baseline incidence values to estimate the health effects over time. A significant correlation was found between PM2.5 and O3 (r =−0.46 in 2019, r =−0.55 in 2020, p < 0.05). The number of premature deaths for all non-accidental causes (27.5 and 24.6), ischemic heart disease (7.3 and 6.3), chronic obstructive pulmonary disease (17 and 19.2), and lung cancer (9.2 and 6.25) attributed to ambient PM2.5 exposure and for respiratory diseases (4.7 and 5.4) for exposure to O3 above 10 µg m−3 for people older than 30-year-old were obtained in 2019 and 2020. The number of years of life lost declined by 11.6% in 2020 and exposure to PM2.5 reduced the life expectancy by 0.58 and 0.45 years, respectively in 2019 and 2020. Compared to 2019, the restrictive measures associated to COVID-19 pandemic led to reduction in PM2.5 (−25.5%) and an increase of O3 concentration (+ 8.0%) in Khorramabad.
Similar content being viewed by others
1 Introduction
Air pollution due to industrialization, urbanization, and population growth is one of the global health issue in the last century (Guan et al. 2016; Landrigan et al. 2018; Neira 2019), especially in developing Middle Eastern countries characterized by dust storms such as Iran (Farzadfar et al. 2022). Therefore, numerous epidemiological studies were conducted about chronic and acute effects of exposure of population to ambient air pollutants (Ostro et al. 2018; Toe et al. 2021). Particulate matters (PM2.5 and PM10), tropospheric ozone (O3) and nitrogen dioxide (NO2) are among the most threatening air pollutants in cities with harmful effects on human health (De Marco et al. 2018; Sicard et al. 2019; Mannucci 2022). Previous studies have reported a strong association between exposure to air pollutants (PM2.5, PM10, O3 and NO2) and cardiovascular and respiratory diseases, and premature mortality (Anderson 2009, Khaniabadi et al. 2018a, Hashemzadeh et al. 2019, Khomenko et al. 2021, Liu et al. 2021, Zhao et al. 2021). Among these air pollutants, PM2.5 and O3 led to e.g., increased cardiorespiratory deaths, asthma exacerbation, and hospital admissions for cardiovascular and respiratory diseases (Christakos and Kolovos 1999; Crouse et al. 2015; Goodman et al. 2015; Amoatey et al. 2019; Yazdi et al. 2019; Sicard et al. 2020). The PM2.5 is linked to increases in respiratory problems, cardiovascular health effects, risk of lung cancer and premature mortality. Climate change and air pollution are closely linked (Sicard et al. 2016a, De Marco et al. 2022a, b) since the source of greenhouse gases and air pollutants are generally similar (O’Donovan et al. 2018; De Marco et al. 2022b). Ozone is the third most important greenhouse gas in terms of radiative forcing, contributing to climate change (Amoatey et al. 2019). Due to the human activities like industrial processing, transportation and energy consumption, the O3 levels increased in the last decades in cities (Siciliano et al. 2020; Zoran et al. 2020; De Marco et al. 2022a), and this increase in cities was intensified during the COVID-19 lockdown (Siciliano et al. 2020; Zoran et al. 2020; Sicard et al. 2020) with negative impacts on human and ecosystems health (Wang et al. 2020; Shin et al. 2021). Research on ambient PM2.5 and O3 and their health effects is conducted in cities such as New York (Kheirbek et al. 2013), Beijing (Xie et al. 2019), Delhi (Amann et al. 2017), Paris (Bressi et al. 2013), Los Angeles (Hasheminassab et al. 2014), Lisbon (Garrett and Casimiro 2011), Rome (Sicard et al. 2019), and Catalonia (Rovira et al. 2020). In Iran, some studies were performed in Ahvaz (Karimi et al. 2019), Tabriz (Barzeghar et al. 2020), Tehran (Faridi et al. 2018), and Shahrekord (Naghan et al. 2022) to assess the effects of PM2.5 and O3 on mortality and morbidity among population. Southwest of Iran (Lorestan Province) has experienced elevated air pollution due to Middle East Dust (MED) storms, road traffic, and increased industrial operations. In recent years, Khorramabad has witnessed similar high exposure to ambient air pollutants such as PM2.5 and O3. The main objectives of the present study were to investigation the health effects of PM2.5 and O3 by concentration–response model, and estimate the years of life lost and loss of life expectancy in 2019 and 2020 in Khorramabad, southwestern city of Iran.
2 Materials and methods
2.1 Study area
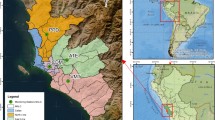
Khorramabad (33°48'N; 48°35'E), with a population of about 540,000 people, is located in Lorestan Province, southwestern of Iran (Fig. 1). The annual mean precipitation and air temperature is 511 mm and 19.2 °C, respectively. Khorramabad is classed under the Köppen climate classification as a hot-summer Mediterranean climate (Daryanoosh et al. 2018). In this region, the main causes of air pollution are the dust storms leading to high concentrations of particulate matters (Kianisadr et al., 2018). In addition, petrochemical industries and road traffic emissions have exacerbated the poorly air quality (Daryanoosh et al. 2018, Kianisadr et al., 2018). The city is surrounded by the high Zagros Mountains (1170 m a.s.l) trapping the ambient air pollutants within the atmospheric boundary layer leading to higher pollution levels (Daryanoosh et al. 2018).
2.2 Data collection
The hourly PM2.5 and O3 concentrations were obtained, from 1st January 2018 to 31st December 2021, from two urban monitoring stations provided by the local Environment Protection Agency under the Iranian Department of Environment (https://www.doe.ir). Then, the hourly data were aggregated to daily averages with at least 75% validated hourly data or non-missing values using Microsoft Excel Package (Khaniabadi and Sicard 2021b).
2.3 Data processing
In this study, we detected outliers within time-series by using Z-scores. The standard cut-off value for finding outliers are Z-scores of ± 3. The PM2.5/O3 ratio was calculated to fully understand the relationship between PM2.5 and O3. This applied ratio can be used as an indicators of air pollution over time to describe the underlying atmospheric processes and to provide further understanding of the spatio-temporal variability of air pollutants. In the risk assessment, exposure is considered equal to air pollutants concentrations at a specific point in space and time (Bogaert et al. 2009; Neisi et al. 2018, Khaniabadi and Sicard 2021b). The concentration–response (C-R) model was developed from current systematic reviews and meta-analysis of various short- and long-term mortality and morbidity health effects for air pollution exposure. For the analysis of exposure by air pollutant, the baseline incidence (BI), relative risk (RR) and the number of people at risk (N) over an area are needed. The BI is the rate of incidence of given health effect in an exposed population and the RR is derived from different published papers and represent the chance of developing air pollution related diseases as a result of exposure per each 10 µg m−3 increase in the air concentration (Sicard et al. 2019). The RR measures the probability of developing a disease related to exposure based on Eq. 1.
where β is a parameter which regulates the amount of RR increasing, X and X0 (µg m−3) also are respectively the measured air pollutant concentrations and background where no health effect recorded (De Marco et al. 2018).
For the quantification of mortality related to ischemic heart disease, chronic obstructive pulmonary disease and lung cancer, integrated exposure–response functions from European cohort studies can be used (Eq. 2).
where z and zcf are respectively the annual mean concentration and the counterfactual PM2.5 concentration below which we assume no additional risk. Also, parameters α, γ and δ are pre-integrated (De Marco et al. 2018; Al-Hemoud et al. 2020; Amoatey et al. 2020; Rovira et al. 2020). Furthermore, the Attributable Proportion (AP %) is the fraction of a health effect that can be statistically associated with the exposure to the air pollutant, c, in a population P(c) (Eq. 3).
where AP is the attributable proportion of the health effect, RR(c) is the relative risk for certain health impacts in category "c" (e.g., residential, or industrial) of exposure obtained from exposure–response functions derived from epidemiological studies (Hadei et al. 2017; Rovira et al. 2020). P(c) is number of individuals at risk (Gurjar et al. 2010; Fattore et al. 2011; Daryanoosh et al. 2017; Khaniabadi et al. 2017a).
For a health effect, the number of cases NCc per 100,000 people at risk attributed to the air pollutant c is calculated as NCc = BI \(*\) AP. The number of excess cases, NEc, attributed to the air pollutant c is calculated as NEc = 10−5 \(*\)[NCc \(*\) N], where N is the number of people at risk exposed to the air pollutant c (De Marco et al. 2018).
SOMO35 (Sum of Ozone Means Over 35 ppb) is a metric for health impact assessment, based on recent epidemiological studies, and recommended by WHO (Aksoyoglu et al. 2014). SOMO35 (in µg m−3 days) is defined as the yearly sum of the daily maximum of 8-h running average for O3 over 35 ppb (i.e., 70 µg m−3) was calculated (Eq. 4).
where Ci is the maximum daily O3 mean concentrations (in µg m−3), and i number of days in a calendar year. Since SOMO35 is sensitive to missing daily O3 concentrations, it is corrected to full annual coverage with actual valid daily concentration levels (Nvalid) according to below equation (Eq. 5).
where, Ntotal = total number of days in a calendar year (365 days in 2019, 366 in 2020), Nvalid = total validated daily O3 mean concentration.
The long-term effects on mortality due to respiratory diseases, according to RR functions, is shown in Eq. 6 for each 10 µg m−3 increase in O3 levels. It is modeled as a log-linear function.
Similarly, for mortality due to respiratory diseases from O3 exposure, the model estimates the integrated exposure–response (IER) function by Eq. 7.
where \({\upbeta }\) is the increase rate of RR. Cmax8 (µg m−3) is the maximum average daily 8-h O3 concentrations and Xo is daily concentration of O3.
The BI is the rate of incidence of a given health outcome in the population. As recommended by WHO, to estimate mortality due to respiratory diseases (M-RD) and all-causes mortality (M-all-cause) attributable to the long-term exposure to PM2.5, we used linear-log function, respectively. Besides, to assess the cause-specific mortality in adults, including ischemic heart disease (M-IHD), chronic obstructive pulmonary disease (M-COPD) and lung cancer (M-LC) among adults more than 30-year-old attributable to PM2.5 exposure, we used the IER function as are described by (Burnett et al. 2014; Héroux et al. 2015; Faridi et al. 2018). Following the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10), J95 and J96 are associated to respiratory mortality, J120-J189, J209-J499, and J690-J700 and J44 codes are associated to COPD, C33 and C34 are correspond to LC, and codes I20 to I25 are related to IHD. Table 1 shows the year-specific BI amounts used in this study for assess of health effects attributed PM2.5 and O3 in Khorramabad, Iran.
2.4 Statistical estimation and years of life lost
Data were analyzed for normal distribution by the Kolmogorov–Smirnov one-sample D-test. The correlation analyses were performed by using the Pearson regression between air pollutants concentrations from January 2019 to January 2020. To estimate the years of life lost (YLL), life table method is used (Hadei et al. 2020) as well as losses of expected life remaining (ELR) in a population. For this, rate of population and deaths are required. The loss of life expectancy using population-weighted was estimated.
3 Results and discussion
3.1 Temporal variability
In 2019 and 2020, the highest PM2.5 (133.6 µg m−3) and O3 (46.1 µg m−3) mean concentrations were recorded in 2019, while lower levels were observed in 2020 (89.7 and 37.7 µg m−3, respectively). The annual PM2.5 mean concentration exceeded the 2021 WHO guideline value for human health protection (5 µg m−3), while O3 did not exceeded the daily 8-h annual mean guideline (100 µg m−3). Figure 2 shows the time series of PM2.5 and O3 level in 2019 (normal condition before COVID-19) and 2020 (during COVID-19 pandemic) in Khorramabad.
In this study, we observed the higher PM2.5 levels within cold season due to activities at home such as high combustion emissions, domestic heating, biomass burning (Amoatey et al. 2020) and dust storms (Amoatey et al. 2021; Goudarzi et al. 2021). In the southwest of Iran, there is encountered with the high levels of particles from MED storms with Arabians' desserts sources (Broomandi et al. 2022) which disperses more during winter season because of higher wind speed (Khaniabadi et al. 2017c, 2019; Omidi Khaniabadi et al. 2019). The O3 levels were higher during warm season due to transportation of air masses, emissions (Wu and Xie 2017), and photo-chemical reaction (Guo et al. 2017) Lei et al. 2019).
3.2 Relationship between PM2.5 and O3
The daily PM2.5 concentrations was well correlated to O3 in 2019 (r = −0.46, p < 0.05) and 2020 (r = −0.55, p < 0.05), with PM2.5/O3 amounts about 4.1 and 2.9, respectively in these two years (Fig. 3). High PM levels leads to a reduced solar radiation (Li et al. 2017; Zhang et al. 2022) leading to a decrease of surface O3 formation (Khaniabadi and Sicard 2021a, Sicard 2021). Furthermore, the heterogeneous chemical processes occurring on PM2.5 surface with O3 are a way for O3 removal by PM2.5 in the atmosphere (Amoatey et al. 2019; Sicard et al. 2019).
Higher PM2.5/O3 ratio in 2019 than 2020 showed the high contribution of traffic road (De Marco et al. 2018), fossil fuels consumption and dust storms (Naghan et al. 2022) leading to rising PM2.5 concentration in the air, reducing solar radiation (Adhikari and Yin 2020). In 2020, COVID-19 lockdown, with restricted activities, led to a reduction in PM2.5 concentrations and to higher O3 levels (Sicard et al. 2020).
3.3 Comparison of mean pollutants and energy consumption
A comparison between the pre-COVID-19 pandemic and COVID-19 period for PM2.5 and O3 mean concentrations in 2018, 2019 and 2020 across Khorramabad, was investigated (Fig. 4). The first overview illustrated, restrictive measures in relation to COVID-19 pandemic led to a reduction in the annual average of PM2.5 by—25.5%. Furthermore, the annual averages O3 in 2020 compared to previous years increased by + 8.0% during COVID-19 year.
(Sicard et al. 2020) showed that O3 increased in Wuhan, Valencia, Nice and Rome higher than previous years 2017–2019, while PM2.5 annual averages was reduced in these cities during 2020. By considering long-time-series, a trends analysis in Marseille showed that the restrictive measures due to COVID-19 led to an actual reduction of 11% in PM2.5 concentrations compared to the time period 2010–2019 (Khaniabadi and Sicard 2021b). Another study showed that O3 was the only pollutant increased over the COVID pandemic, and then decreased by 20% post-COVID with government monitoring (Bhatti et al. 2021). In Almaty, Kazakhstan (Kerimray et al. 2020), the annual averages of PM2.5 during 2019 and 2020 were respectively 40 and 31 µg m−3, in which the concentration was reduced during COVID-19 at 2020 almost−29%.
The yearly comparative of fuel consumption including gasoline and natural gas pre-pandemic (2018 and 2019) and during-COVID-19 (2020) showed a lowering in the cost of consumption fuels during the COVID-19. The variation rate for gasoline (m3 year−1) and natural gas (in Mm3 year−1) lead to a reduction in cost for people about −13.06% and −12.56%, respectively in 2020 (Fig. 4). There was a positive association between PM2.5 concentration and gasoline and natural gas consumption (p < 0.05), while a negatively association with O3 concentration (p < 0.05) was performed.
The cost-reduction indicates that the higher air quality has a positive association with human activity, lower traffic and the closure of factories which all causes reduce consumption of energy (Tian et al. 2021) and provides a better air quality (Agami and Dayan 2021) in the different areas within the world. A comparative study on fuel consumption in Israel indicated that with extensive COVID-19 in the world, the consumption of gasoline and natural gas was reduced −22.5% and −10% respectively than the same period within 2019. In Turkey, (Güngör et al. 2021) demonstrates gasoline consumption during COVID-19 was lower than the same time in 2019.
3.4 Health impact of PM2.5 and O3 and the years of life lost
The number of premature deaths for non-accidental causes for PM2.5 above 10 µg m−3 ranged from 7.3 to 27.5, and from 6.2 to 24.6 per 105 inhabitants in 2019 and 2020, respectively (Table 2). The number of M-RD for long-term exposure to O3 in 2019 and 2020 were estimated at 4.7 and 5.4 per 105 inhabitants, respectively. The main causes of non-accidental mortality among 105 people due to exposure to PM2.5 was M-COPD for people older than 30-year-old. The rate of mortality due to exposure to PM2.5 decreased 7.6% per 105 inhabitants, while for O3 an increase of 14.8% M-RD per 105 inhabitants was found in 2019 and 2020, respectively. The highest and the lowest annual change in non-accidental deaths for PM2.5 were observed for M-COPD (+ 1.8 deaths per 105 inhabitants) and M-LC (−2.95 deaths per 105 inhabitants). Also, M-RD increased (+ 0.7 deaths per 105 inhabitants) in 2020 than 2019 due to exposure to different O3 level. The number of premature deaths in 2020, excluding COVID-19 impact, for PM2.5 exposure was M-all-cause = 20.4, IHD = 5.4, COPD = 14.4, LC = 6, and for O3 exposure M-RD = 4.0 per 105 inhabitants at risk.
In Ahvaz with a population of about 1.3 million people, M-all-cause and M-RD, respectively for PM2.5 and O3 exposure were 240 and 2.72 for adults ≥ 30 year-old (Karimi et al. 2019). In Tehran (Faridi et al. 2018) showed the number of premature M-COPD increased by 57% from 2010 to 2015, while M-IHD was reduced by 28%. With a 10 µg m−3 increase in the rate of daily PM2.5 exposure, 1.04% increased mortality for non-accidental causes worldwide (Atkinson et al. 2014). The MED storms is one of the most important reasons which caused high levels of particles in air, and subsequence the increase of mortality and morbidity in west and southwest of Iran (Khaniabadi et al. 2017d, Khaniabadi et al. 2018b). In Marseille (South of France), the number of non-accidental M-all-cause decreased by 1.15 per 105 people approximately (Khaniabadi and Sicard 2021a). The surface O3 as a global serious problem, has considered in various internal projects in Europe, the United States, and Asia (Sicard et al. 2017). Another study by (Barzeghar et al. 2020) showed M-RD O3 as SOMO35 increased in Tabriz within 2018 than previous years. The estimated premature M-RD due to exposure to atmospheric O3 levels in the at-risk population was 143 in 2019 cases while it was 242 in 2013 in Portugal (Brito et al. 2022).
The highest YLL is 1456 in 2019 and then declined by 11.6% in 2020 (Table 3). Results showed exposure to air PM2.5 in 2019 and 2020 reduced the life expectancy by 0.58 and 0.45 years during each year, respectively. The loss of life expectancy due to PM2.5 exposure in Iran was estimated at 0.43–1.87 years (Hadei et al. 2020). The YLL attributable to PM2.5 for individuals higher than 30 year-old was decreased by 30% during 10 years (2006–2015) in Tehran (Faridi et al. 2018).
4 Conclusion
In this study, we investigated the temporal trends in PM2.5 and O3 concentrations in a southwestern capital city of Iran. The main source of PM2.5 as most serious health risk within southwest of Iran is MED storms from Arabian countries (Karimi et al. 2019; Goudarzi et al. 2021). This study has conducted with a methodology from high-quality research, but there are still limitations. The interactions between air pollutants and their information are not available, however the health effects are focused on a single pollutant without considering the simultaneous exposure to the multiple pollutants. This study only reflects outdoor exposure while people spend longer indoors (Guan et al. 2021b). A comprehensive estimation of indoor air pollution health effects by (Hu et al. 2020) showed that the indoor exposure accounted for 68% and 34% of that to ambient PM2.5 and O3. Therefore, this study may overestimate the health effects to a certain extent. Our approach assumes that air concentrations measured at the central monitoring point are representative of the exposure of all people living in a city (Khaniabadi et al. 2017b; Guan et al. 2021a). In general, the health effect findings based on daily averaged not suitable for direct comparison (Guan et al. 2021b).
Between 2018 and 2020, PM2.5 mean concentrations ranged from 27.7 to 41.2 to µg m−3, while O3 levels ranged from 14.2 to 15.7 µg m−3. Highest PM2.5 and O3 levels were observed during cold and warm seasons with good coefficient correlation in 2019 and 2020. The COVID-19 lockdown reduced PM2.5 concentrations (−25%) and related mortality (−7.5%) but O3 levels increased (+ 8%), as well as the O3-related number of premature deaths (+ 15%). PM2.5 concentrations is well correlated to gasoline and natural gas consumption, and negatively correlated to O3, leading to a reduction in cost for people (−13.1% and−12.6% respectively in 2020), compared to previous years. In 2019 the number of premature deaths related to PM2.5 (7.3 to 27.5 per 105 people) and O3 (4.7 per 105 people) concentrations changed to 6.3 to 24.6 per 105 people and 5.4 per 105 people in 2020. Our results showed exposure to PM2.5 reduced the life expectancy by 0.58 and 0.45 year in 2019 and 2020, respectively. Previous studies provided quantitative estimates of the ability of green infrastructure to ameliorate urban air quality (e.g., PM2.5, O3) at city scale worldwide (Adhikari and Yin 2020, Khaniabadi and Sicard 2021a).
Abbreviations
- RR:
-
Relative risk
- BI:
-
Baseline incidence
- AP:
-
Attributable proportion
- NC:
-
Number of cases
- NE:
-
Number of excess cases
- SOMO35:
-
Sum of O3 means over 35 ppb
- RD:
-
Respiratory diseases
- COPD:
-
Chronic obstructive pulmonary disease
- LC:
-
Lung cancer
- IHD:
-
Ischemic heart disease
- IER:
-
Integrated exposure–response
- YLL:
-
Years of life lost
- ELR:
-
Expected life remaining
References
Adhikari A, Yin J (2020) Short-term effects of ambient ozone, PM2.5, and meteorological factors on COVID-19 confirmed cases and deaths in queens, new york. Int J Environ Res Public Health 17:4047
Agami S, Dayan U (2021) Impact of the first induced COVID-19 lockdown on air quality in Israel. Atmos Environ 262:118627
Aksoyoglu S, Keller J, Ciarelli G, Prévôt AS, Baltensperger U (2014) A model study on changes of european and swiss particulate matter, ozone and nitrogen deposition between 1990 and 2020 due to the revised gothenburg protocol. Atmos Chem Phys 14:13081–13095
Al-Hemoud A, Gasana J, Alajeel A, Alhamoud E, Al-Shatti A, Al-Khayat A (2020) Ambient exposure of O3 and NO2 and associated health risk in Kuwait. Environ Sci Pollut Res 28:1–10
Amann M, Purohit P, Bhanarkar AD, Bertok I, Borken-Kleefeld J, Cofala J, Heyes C, Kiesewetter G, Klimont Z, Liu J (2017) Managing future air quality in megacities: a case study for delhi. Atmos Environ 161:99–111
Amoatey P, Takdastan A, Sicard P, Hopke PK, Baawain M, Omidvarborna H, Allahyari S, Esmaeilzadeh A, De Marco A, Khanaibadi YO (2019) Short and long-term impacts of ambient ozone on health in ahvaz Iran. Human Ecol Risk Assess Int J 25:1336
Amoatey P, Sicard P, De Marco A, Khaniabadi YO (2020) Long-term exposure to ambient PM2.5 and impacts on health in rome Italy. Clin Epidemiol Glob Health 8:531–535
Amoatey P, Khaniabadi YO, Sicard P, Siddiqi SA, De Marco A, Sulaiman H (2021) Temporal incidence and prevalence of bronchitis and morbidities from exposure to ambient PM2.5 and PM10. Environ Justice 14:267–276
Anderson HR (2009) Air pollution and mortality: a history. Atmos Environ 43:142–152
Atkinson R, Kang S, Anderson H, Mills I, Walton H (2014) Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax 69:660–665
Barzeghar V, Sarbakhsh P, Hassanvand MS, Faridi S, Gholampour A (2020) Long-term trend of ambient air PM10, PM2.5, and O3 and their health effects in tabriz city, Iran, during 2006–2017. Sustain Cities Soc 54:101988
Bhatti UA, Zeeshan Z, Nizamani MM, Bazai S, Yu Z, Yuan L (2021) Assessing the change of ambient air quality patterns in jiangsu province of china pre-to post-COVID-19. Chemosphere 288:132569
Bogaert P, Christakos G, Jerrett M, Yu H-L (2009) Spatiotemporal modelling of ozone distribution in the state of california. Atmos Environ 43:2471–2480
Bressi M, Sciare J, Ghersi V, Bonnaire N, Nicolas J, Petit J-E, Moukhtar S, Rosso A, Mihalopoulos N, Féron A (2013) A one-year comprehensive chemical characterisation of fine aerosol (PM2.5) at urban, suburban and rural background sites in the region of Paris (France). Atmos Chem Phys 13:7825–7844
Brito J, Bernardo A, Gonçalves LL (2022) Atmospheric pollution and mortality in portugal: quantitative assessment of the environmental burden of disease using the AirQ+ model. Sci Total Environ 285:152964
Broomandi P, Crape B, Jahanbakhshi A, Janatian N, Nikfal A, Tamjidi M, Kim JR, Middleton N, Karaca F (2022) Assessment of the association between dust storms and COVID-19 infection rate in southwest iran. Environ Sci Pollut Res 29:1–20
Burnett RT, Pope CA III, Ezzati M, Olives C, Lim SS, Mehta S, Shin HH, Singh G, Hubbell B, Brauer M (2014) An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ Health Perspect 122:397–403
Christakos G, Kolovos A (1999) A study of the spatiotemporal health impacts of ozone exposure. J Eposure Sci Environ Epidemiol 9:322–335
Crouse DL, Peters PA, Hystad P, Brook JR, van Donkelaar A, Martin RV, Villeneuve PJ, Jerrett M, Goldberg MS, Pope CA III (2015) Ambient PM2.5, O3, and NO2 exposures and associations with mortality over 16 years of follow-up in the canadian census health and environment cohort (CanCHEC). Environ Health Perspect 123:1180–1186
Daryanoosh M, Goudarzi G, Rashidi R, Keishams F, Hopke PK, Mohammadi MJ, Nourmoradi H, Sicard P, Takdastan A, Vosoughi M, Veysi M, Kianizadeh M, Khaniabadi YO (2017) Risk of morbidity attributed to ambient PM10 in the western cities of Iran. Toxin Rev. https://doi.org/10.1080/15569543.15562017.11370602
Daryanoosh M, Goudarzi G, Rashidi R, Keishams F, Hopke PK, Mohammadi MJ, Nourmoradi H, Sicard P, Takdastan A, Vosoughi M (2018) Risk of morbidity attributed to ambient PM10 in the western cities of Iran. Toxin Rev 37:313–318
De Marco A, Amoatey P, Khaniabadi YO, Sicard P, Hopke PK (2018) Mortality and morbidity for cardiopulmonary diseases attributed to PM2.5 exposure in the metropolis of rome Italy. Eur J Intern Med 57:49–57
De Marco A, Garcia-Gomez H, Collalti A, Khaniabadi YO, Feng Z, Proietti C, Sicard P, Vitale M, Anav A, Paoletti E (2022) Ozone modelling and mapping for risk assessment: an overview of different approaches for human and ecosystems health. Environ Res 211:113048
De Marco A, Sicard P, Feng Z, Agathokleous E, Alonso R, Araminiene V, Augustaitis A, Badea O, Beasley JC, Branquinho C (2022) Strategic roadmap to assess forest vulnerability under air pollution and climate change. Glob Ch Biol. https://doi.org/10.1111/gcb.16278
Faridi S, Shamsipour M, Krzyzanowski M, Künzli N, Amini H, Azimi F, Malkawi M, Momeniha F, Gholampour A, Hassanvand MS (2018) Long-term trends and health impact of PM2.5 and O3 in tehran, iran, 2006–2015. Environ Int 114:37–49
Farzadfar F, Naghavi M, Sepanlou SG, Moghaddam SS, Dangel WJ, Weaver ND, Aminorroaya A, Azadnajafabad S, Koolaji S, Mohammadi E (2022) Health system performance in Iran: a systematic analysis for the global burden of disease study 2019. Lancet 399:1625–1645
Fattore E, Paiano V, Borgini A, Tittarelli A, Bertoldi M, Crosignani P, Fanelli R (2011) Human health risk in relation to air quality in two municipalities in an industrialized area of northern italy. Environ Res 111:1321–1327
Garrett P, Casimiro E (2011) Short-term effect of fine particulate matter (PM2.5) and ozone on daily mortality in lisbon. Port Environ Sci Pollut Res 18:1585–1592
Goodman JE, Prueitt RL, Sax SN, Pizzurro DM, Lynch HN, Zu K, Venditti FJ (2015) Ozone exposure and systemic biomarkers: evaluation of evidence for adverse cardiovascular health impacts. Crit Rev Toxicol 45:412–452
Goudarzi G, Hopke PK, Yazdani M (2021) Forecasting PM2.5 concentration using artificial neural network and its health effects in ahvaz Iran. Chemosphere 283:131285
Guan W-J, Zheng X-Y, Chung KF, Zhong N-S (2016) Impact of air pollution on the burden of chronic respiratory diseases in china: time for urgent action. Lancet 388:1939–1951
Guan Y, Xiao Y, Rong B, Zhang N, Chu C (2021) Long-term health impacts attributable to PM2.5 and ozone pollution in china’s most polluted region during 2015–2020. J Clean Prod 321:128970
Guan Y, Xiao Y, Wang Y, Zhang N, Chu C (2021) Assessing the health impacts attributable to PM2.5 and ozone pollution in 338 chinese cities from 2015 to 2020. Environ Pollut 287:117623
Güngör BO, Ertuğrul HM, Soytaş U (2021) Impact of Covid-19 outbreak on turkish gasoline consumption. Technol Forecast Soc Chang 166:120637
Guo H, Ling Z, Cheng H, Simpson I, Lyu X, Wang X, Shao M, Lu H, Ayoko G, Zhang Y (2017) Tropospheric volatile organic compounds in china. Sci Total Environ 574:1021–1043
Gurjar B, Jain A, Sharma A, Agarwal A, Gupta P, Nagpure A, Lelieveld J (2010) Human health risks in megacities due to air pollution. Atmos Environ 44:4606–4613
Hadei M, Nazari SSH, Eslami A, Khosravi A, Yarahmadi M, Naghdali Z, Shahsavani A (2017) Distribution and number of ischemic heart disease (IHD) and stroke deaths due to chronic exposure to PM2.5 in 10 cities of Iran (2013–2015); an AirQ+ modelling. J Air Pollut Health 2:129–136
Hadei M, Shahsavani A, Krzyzanowski M, Querol X, Stafoggia M, Nazari SSH, Jafari AJ, Yarahmadi M, Kermani M, Khosravi A (2020) Burden of mortality attributed to PM2.5 exposure in cities of iran; contribution of shortterm pollution peaks. Atmos Environ 224:117365
Hasheminassab S, Daher N, Ostro BD, Sioutas C (2014) Long-term source apportionment of ambient fine particulate matter (PM2. 5) in the los angeles basin: a focus on emissions reduction from vehicular sources. Environ Pollut 193:54–64
Hashemzadeh B, Idani E, Goudarzi G, Ankali KA, Sakhvidi MJZ, Babaei AA, Hashemzadeh H, Vosoughi M, Mohammadi MJ, Neisi A (2019) Effects of PM2. 5 and NO2 on the 8-isoprostane and lung function indices of FVC and FEV1 in students of ahvaz city Iran. Saudi J Biol Sci 26:473–480
Héroux M-E, Anderson HR, Atkinson R, Brunekreef B, Cohen A, Forastiere F, Hurley F, Katsouyanni K, Krewski D, Krzyzanowski M (2015) Quantifying the health impacts of ambient air pollutants: recommendations of a WHO/Europe project. Int J Public Health 60:619–627
Hu Y, Yao M, Liu Y, Zhao B (2020) Personal exposure to ambient PM2.5, PM10, O3, NO2, and SO2 for different populations in 31 chinese provinces. Environ Int 144:106018
Karimi A, Shirmardi M, Hadeie M, Birgani YT, Neisi A, Takdastan A, Goudarzi G (2019) Concentrations and health effects of short- and long-term exposure to PM25, NO2, and O3 in ambient air of Ahvaz city, Iran (2014–2017). Ecotoxicology and Environmental Safety 180: 542–548
Kerimray A, Baimatova N, Ibragimova OP, Bukenov B, Kenessov B, Plotitsyn P, Karaca F (2020) Assessing air quality changes in large cities during COVID-19 lockdowns: the impacts of traffic-free urban conditions in almaty. Kaz Sci Total Environ 730:139179
Khaniabadi YO, Sicard P (2021) A 10-year assessment of ambient fine particles and related health endpoints in a large mediterranean city. Chemosphere 278:130502
Khaniabadi YO, Goudarzi G, Daryanoosh SM, Borgini A, Tittarelli A, De Marco A (2017a) Exposure to PM10, NO2, and O3 and impacts on human health. Environ Sci Pollut Res 24:2781–2789
Khaniabadi YO, Hopke PK, Goudarzi G, Daryanoosh SM, Jourvand M, Basiri H (2017b) Cardiopulmonary mortality and COPD attributed to ambient ozone. Environ Res 152:336–341
Khaniabadi YO, Daryanoosh SM, Amrane A, Polosa R, Hopke PK, Goudarzi G, Mohammadi MJ, Sicard P, Armin H (2017c) Impact of middle eastern dust storms on human health. Atmos Pollut Res 8:606–613
Khaniabadi YO, Fanelli R, De Marco A, Daryanoosh SM, Kloog I, Hopke PK, Conti GO, Ferrante M, Mohammadi MJ, Babaei AA (2017d) Hospital admissions in Iran for cardiovascular and respiratory diseases attributed to the middle eastern dust storms. Environ Sci Pollut Res 24:16860–16868
Khaniabadi YO, Daryanoosh M, Sicard P, Takdastan A, Hopke PK, Esmaeili S, De Marco A, Rashidi R (2018) Chronic obstructive pulmonary diseases related to outdoor PM10, O3, SO2, and NO2 in a heavily polluted megacity of Iran. Environ Sci Pollut Res 25:17726–17734
Khaniabadi YO, Sicard P, Takdastan A, Hopke PK, Taiwo AM, Khaniabadi FO, De Marco A, Daryanoosh M (2019) Mortality and morbidity due to ambient air pollution in Iran. Clin Epidemiol Glob Health 7:222–227
Kheirbek I, Wheeler K, Walters S, Kass D, Matte T (2013) PM2.5 and ozone health impacts and disparities in new york city: sensitivity to spatial and temporal resolution. Air Qual Atmos Health 6:473–486
Khomenko S, Cirach M, Pereira-Barboza E, Mueller N, Barrera-Gómez J, Rojas-Rueda D, de Hoogh K, Hoek G, Nieuwenhuijsen M (2021) Premature mortality due to air pollution in European cities: a health impact assessment. Lancet Planet Health 5:e121–e134
Landrigan PJ, Fuller R, Acosta NJ, Adeyi O, Arnold R, Baldé AB, Bertollini R, Bose-O’Reilly S, Boufford JI, Breysse PN (2018) The lancet commission on pollution and health. Lancet 391:462–512
Li M, Wang T, Xie M, Zhuang B, Li S, Han Y, Chen P (2017) Impacts of aerosol-radiation feedback on local air quality during a severe haze episode in nanjing megacity, eastern china. Tellus B Chem Phys Meteorol 69:1339548
Liu Y, Pan J, Fan C, Xu R, Wang Y, Xu C, Xie S, Zhang H, Cui X, Peng Z (2021) Short-term exposure to ambient air pollution and mortality from myocardial infarction. J Am Coll Cardiol 77:271–281
Mannucci PM (2022) Cardiovascular health and ambient air pollution: lower is not enough. Eur J Prev Cardiol 29:1200–1201
Naghan DJ, Neisi A, Goudarzi G, Dastoorpoor M, Fadaei A, Angali KA (2022) Estimation of the effects PM2.5, NO2, O3 pollutants on the health of shahrekord residents based on AirQ+ software during (2012–2018). Toxicol Rep 9:842
Neira MP (2019) Air pollution and human health: a comment from the World Health Organization. Ann Glob Health. https://doi.org/10.5334/aogh.2712
Neisi A, Vosoughi M, Shirmardi M, Idani E, Goudarzi G, Hazrati S, Mohammadi MJ, Asadi A, Asgharnia H, Hashemzadeh B (2018) Concentration of air pollutants as toxic matter in urban and rural areas of Ahvaz. Toxin Rev 37:243–250
O’Donovan MR, Gapp C, Stein C (2018) Burden of disease studies in the WHO european region—a mapping exercise. Eur J Pub Health 28:773–778
Omidi Khaniabadi Y, Sicard P, Omidi Khaniabadi A, Mohammadinejad S, Keishams F, Takdastan A, Najafi A, De Marco A, Daryanoosh M (2019) Air quality modeling for health risk assessment of ambient PM10, PM2. 5 and SO2 in Iran. Hum Ecol Risk Assess Int J 25:1298–1310
Ostro B, Spadaro JV, Gumy S, Mudu P, Awe Y, Forastiere F, Peters A (2018) Assessing the recent estimates of the global burden of disease for ambient air pollution: methodological changes and implications for low-and middle-income countries. Environ Res 166:713–725
Rovira J, Domingo JL, Schuhmacher M (2020) Air quality, health impacts and burden of disease due to air pollution (PM10, PM2.5, NO2 and O3): Application of AirQ+ model to the Camp de Tarragona County (Catalonia, Spain). Sci Total Environ 703:135538
Shin HH, Gogna P, Maquiling A, Parajuli RP, Haque L, Burr B (2021) Comparison of hospitalization and mortality associated with short-term exposure to ambient ozone and PM2.5 in canada. Chemosphere 265:128683
Sicard P (2021) Ground-level ozone over time: an observation-based global overview. Curr Opin Environ Sci Health 19:100226
Sicard P, Khaniabadi YO, Perez S, Gualtieri M, De Marco A (2019) Effect of O3, PM10 and PM2. 5 on cardiovascular and respiratory diseases in cities of France, Iran and Italy. Environ Sci Pollut Res 26:32645–32665
Sicard P, De Marco A, Agathokleous E, Feng Z, Xu X, Paoletti E, Rodriguez JJD, Calatayud V (2020) Amplified ozone pollution in cities during the COVID-19 lockdown. Sci Total Environ 735:139542
Siciliano B, Dantas G, da Silva CM, Arbilla G (2020) Increased ozone levels during the COVID-19 lockdown: analysis for the city of Rio de Janeiro. Braz Sci Total Environ 737:139765
Tian X, An C, Chen Z, Tian Z (2021) Assessing the impact of COVID-19 pandemic on urban transportation and air quality in canada. Sci Total Environ 765:144270
Toe S, Nagy M, Albar Z, Nazzinda R, Sattar A, Musiime V, Etajak S, Walyawula F, McComsey GA, Atuyambe L (2021) Ambient air pollution and cardiovascular disease in Ugandan adolescents with perinatally acquired HIV: a cross-sectional study. Lancet Glob Health 9:S21
Wang Y, Wild O, Chen X, Wu Q, Gao M, Chen H, Qi Y, Wang Z (2020) Health impacts of long-term ozone exposure in china over 2013–2017. Environ Int 144:106030
Wu R, Xie S (2017) Spatial distribution of ozone formation in china derived from emissions of speciated volatile organic compounds. Environ Sci Technol 51:2574–2583
Xie Y, Dai H, Zhang Y, Wu Y, Hanaoka T, Masui T (2019) Comparison of health and economic impacts of PM2.5 and ozone pollution in china. Environ Int 130:104881
Yazdi MD, Wang Y, Di Q, Zanobetti A, Schwartz J (2019) Long-term exposure to PM2.5 and ozone and hospital admissions of medicare participants in the southeast USA. Environ Int 130:104879
Zhang W, Li W, An X, Zhao Y, Sheng L, Hai S, Li X, Wang F, Zi Z, Chu M (2022) Numerical study of the amplification effects of cold-front passage on air pollution over the north china plain. Sci Total Environ 833:155231
Zhao S, Liu S, Hou X, Sun Y, Beazley R (2021) Air pollution and cause-specific mortality: a comparative study of urban and rural areas in china. Chemosphere 262:127884
Zoran MA, Savastru RS, Savastru DM, Tautan MN (2020) Assessing the relationship between ground levels of ozone (O3) and nitrogen dioxide (NO2) with coronavirus (COVID-19) in milan Italy. Sci Total Environ 740:140005
Acknowledgements
Thanks to Lorestan University of Medical Sciences for financial supporting. The current study has been approved by the ethical committee Lorestan University of Medical Sciences (IR.LUMS.REC.1400.184).
Funding
This work is supported by Lorestan University of Medical Sciences (Grant number IR.LUMS.REC.1400.184).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Conflict of interest There is no conflict of interest in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rashidi, R., Khaniabadi, Y.O., Sicard, P. et al. Ambient PM2.5 and O3 pollution and health impacts in Iranian megacity. Stoch Environ Res Risk Assess 37, 175–184 (2023). https://doi.org/10.1007/s00477-022-02286-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00477-022-02286-z