Abstract
Background
Tumor lysis syndrome (TLS) and its most serious complication, acute kidney injury (AKI) are one of the emergency conditions in onco-hematology. It is difficult to predict the degree of kidney involvement. Therefore, we studied children with leukemia and lymphoma treated in four Hungarian tertiary centers (inpatient university clinics) retrospectively (2006–2016) from a nephrological aspect.
Method
Data of 31 pediatric patients were obtained from electronic- and paper-based medical records. Physical status, laboratory test results, treatments, and outcomes were assessed. Patients were analyzed according to both “traditional” TLS groupings, as laboratory TLS or clinical TLS, and nephrological aspect based on pRIFLE classification, as mild or severe AKI.
Results
Significant differences were found between the changes in parameters of phosphate homeostasis and urea levels in both classifications. Compared to age-specific normal phosphate ranges, before the development of TLS, hypophosphatemia was common (19/31 cases), while in the post-TLS period, hyperphosphatemia was observed (26/31 cases) most frequently. The rate of daily change in serum phosphate level was significant in the nephrological subgroups, but peaks of serum phosphate level show only a moderate increase. The calculated cut-off value of daily serum phosphate level increased before AKI was 0.32 mmol/L per ROC analysis for severe TLS–AKI. The 24-h urinalysis data of eight patients revealed transiently increased phosphate excretion only in those patients with TLS in whom serum phosphate was elevated in parallel.
Conclusion
Daily serum phosphate level increase can serve as a prognostic factor for the severity of pediatric TLS, as well as predict the severity of kidney involvement.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information
Similar content being viewed by others
Introduction
Tumor lysis syndrome (TLS) is characterized by hyperuricemia, hyperphosphatemia, hyperkalemia, and hypocalcemia [1]. According to the Howard modification of the Cairo–Bishop criteria, at least two of the abovementioned parameters exceed the normal ranges with a margin of 25% at the same time (in 24 h) [2, 3].
Clinically significant TLS (CTLS) is known to be associated with high morbidity and mortality, as its rapid progression may result in severe organ damage including kidney impairment, seizures, cardiac arrhythmias, pulmonary edema, or even death [3, 4]. Acute kidney injury (AKI) is one of the most common complications and an important predictor of short- and long-term mortality [5].
The pathomechanism of TLS is complex, consisting of both crystal-dependent nephropathy and certain crystal-independent mechanisms, which can cause endothelial damage and microvascular dysfunction [6, 7]. Glomerular filtration rate (GFR) and renal blood flow were frequently found reduced by approximately 50% during kidney examination already in cases with mild hyperuricemia, which may indicate early kidney involvement [8,9,10].
The current TLS management of the Hungarian Pediatric Oncology Group (HPOG) corresponded with the guideline of the “British Hematology Standards Committee,” in which patients were grouped on the basis of risk classification [11, 12]. In “low-risk” patients the guideline-recommended aggressive hydration with the use of allopurinol and a “watch and wait” strategy, even though this approach might only decelerate the TLS-related pathological processes [13]. One of the main elements of conservative treatment in the “intermediate” and “high” risk TLS patients is the use of recombinant urate-oxidase (Rasburicase®) (rUO), which is capable of removing uric acid (UA) effectively [14, 15]. However, the influence of rUO treatment and UA levels achieved by rUO treatment is controversial in terms of developing kidney failure. Several studies have come to result that the effect of rUO treatment on risk for AKI was not significant according to multivariate modeling [16, 17]. The use of rUO early in the course of AKI may mitigate further kidney damage, especially in mild AKI, where regeneration is also faster with the application of rUO, but the outcome of severe AKI may not be affected significantly [14, 18]. The pediatric dose of rUO treatment also differs in the literature due to its high cost and severe side effect profile. There was no significant difference between the fixed dose and doses optimized for body weight in terms of the incidence of AKI [19].
In severe cases, the treatment should be combined with kidney replacement therapy (KRT) [11, 20], but there is no consensus in the modality and timing of KRT [21, 22]. Early introduction of KRT has been proven to be kidney protective with better long-term kidney survival [20, 22]. In comparative studies of early and late initiation of dialysis, the increase in the frequency of cannula sepsis was highlighted as a major disadvantage [22, 23].
Recently, in parallel with the increasing use of highly effective anti-cancer treatments, the incidence of TLS has been growing [4, 14, 24]. Early recognition of AKI in TLS is important in guiding further management of these patients.
The objective of our multicenter study was to perform a comprehensive analysis of patients with TLS from a nephrological perspective. We analyzed the incidence and characteristics of AKI among pediatric patients with TLS on the basis of clinical and laboratory data, including kidney function tests, electrolyte levels, and 24-h urine samples. We aimed to select the best-performing conventional biomarker for the prognosis and severity of pediatric TLS and predict the development of AKI.
Material and methods
Study design
This retrospective clinical investigation was carried out between 2006 and 2016 and included children with leukemia and non-Hodgkin lymphoma (NHL) with at least two laboratory abnormalities characteristic of TLS according to the Cairo–Bishop criteria [2, 15]. The study was performed in tertiary pediatric hematology-oncology divisions at four university hospitals (Semmelweis University, Budapest; University of Debrecen, Debrecen; University of Pécs, Pécs; and University of Szeged, Szeged) in Hungary. Data were obtained from the patients’ paper- and electronic-based medical documentation and from the database of the Hungarian Pediatric Tumor Registry.
Patients
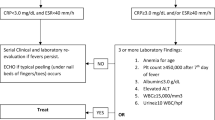
All children with leukemia and lymphoma, who were treated in the above centers during the study period (01.01.2006–12.31.2016) were included in our investigation, a total of 913 children. Sixty-four patients were selected based on predefined laboratory criteria of TLS. Twenty-eight children were excluded from further analysis as laboratory changes were attributed to potential causes different from TLS, and an additional 5 further patients were excluded because of incomplete documentation (Fig. 1).
The remaining 31 patients were categorized according to two classifications, i.e., “traditional” TLS (Laboratory TLS (LTLS) and CTLS) and nephrological point of view based on the calculated GFR values and urine volume using the pRIFLE classification. In the grouping, we distinguished between no and mild kidney damage (0,R,I-pRIFLE) and severe kidney damage (F-pRIFLE) (Fig. 1.)
Patients’ characteristics were further analyzed on the basis of the general condition of patients, their medication, laboratory findings, and applied clinical interventions. The nephrotoxic drug burden of patients was determined according to the publication by Ehrmann et al. [25]. This calculation was applied retrospectively in all patients for the 5 days preceding the development of TLS.
Treatment of patients with TLS–AKI was performed according to international recommendations, also accepted by HPOG, using a multidisciplinary approach [11, 26]. The university treatment protocols were the same in terms of hydration and allopurinol use, as well as KRT indication.
As the examination period was relatively long, the treatment of patients included was not completely uniform in terms of rUO use. Over the 10 years of the study period, about 50% of the patients received rUO.
Clinical and laboratory characteristics and treatments of patients are included in Table 1.
Nephrological assessment
The GFR was calculated according to Bedside Schwarz Eq. 2009 formula [27]. Assessment of changes in serum and urine phosphate levels was based on age-specific normal serum phosphate ranges and 24-h collected urine findings.
The age-specific normal serum phosphate range [28]:
Age (years) | 0–0.5 | 0.5–1 | 1–5 | 6–12 | 13–20 |
|---|---|---|---|---|---|
Serum phosphate (mg/dL) | 5.2–8.4 | 5–7.8 | 4.5–6.5 | 3.6–5.8 | 2.3–4.5 |
Data of 24-h urine examination were available in 8/31 patients. Urine parameters and their formulas are as follows [29,30,31]:
-
u(urine) Phosphate/Cr (creatinine) = u Phosphate/uCr ratio (mmol/mmol)
-
The ratio of the maximum rate of renal tubular reabsorption of phosphate to GFR:TmPO4/GFR = serum (se) Phosphate – (u Phosphate/uCr) ×seCr
-
Fractional excretion (FE) of Phosphate% = [u Phosphate (mg/dL)/se Phosphate (mg/dL)] × [seCr (mg/dL)/uCr (mg/dL)] × 100
-
Ca (calcium) /Cr ratio (mmol/mmol) = [24-h uCa × seCr]/[seCa × 24-h uCr]
-
Ca excretion (mg/kg/24 h)
-
FE of Sodium (Na) (FENa)% = 100 × (seCr (mg/dL) × uNa)/(seNa × uCr (mg/dL)
Statistical analyses
Statistical analyses were conducted using SPSS 24.0 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.). Categorical values are presented as case numbers (n) and percentages (%), and continuous data are expressed as median values (Med) with the corresponding interquartile range (IQR). We compared the background characteristics of the subgroups with the use of univariate analyses using Mann–Whitney and Fisher exact tests, taking into account the low sample size and non-normality of the data.
We investigated the discriminatory ability of clinically important laboratory markers for TLS by using the ROC analysis for the severe TLS–AKI (pRIFLE: F) subgroup and checked the cut-off values.
Results
Patient characteristics
The incidence of TLS in our study was 31/913 patients (3.4%). We observed nine patients with spontaneous TLS. There were 6/31 LTLS (19%) and 25/31 CTLS (80%). In terms of gender, a male predominance was observed, and the median age increased in parallel with the severity of TLS. According to the Cairo–Bishop criteria, hyperphosphatemia was present in 28 patients (28/31), followed by hyperuricemia (21/31), hypocalcemia (19/31), and hyperkalemia (6/31).
The most common laboratory anomaly associated with TLS was elevated serum Creatinine indicating kidney injury (25/31), based on the definition of CTLS. Nine of 25 patients (36%) required KRT due to hyperphosphatemia (7/9) and oliguria (2/9).
The LTLS group mostly consisted of patients with mild kidney impairment (pRIFLE: 0, R); the CTLS group comprised patients with moderate AKI (pRIFLE: I) and severe (pRIFLE: F) AKI. There was only one patient who did not meet the criteria of KRT but had severe AKI (pRIFLE: F).
Based on the statistical analysis of the “traditional” classification, we found a significant difference in the total number of clinical symptoms of TLS, as well as the distribution of patients in the risk groups and under a nephrotoxic drug burden. Among the main laboratory parameters some, such as the serum levels of urea (both at the onset of TLS and during kidney failure) and some parameters representing phosphate homeostasis, showed significant differences.
Hypophosphatemia on days − 3 to − 1 preceding the onset of TLS was detectable altogether in 19/31 cases (61%), and in parallel with the severity of the disease, an increasingly severe initial hypophosphatemia was observed. As expected, hyperphosphatemia was detected in most patients (83%) (Table 1).
Analysis of kidney failure
Patients were divided into two groups: the severe AKI (pRIFLE: F) group and the mild AKI (pRIFLE: 0, R, I) group, in order to analyze from the nephrological aspect.
Statistical analysis of TLS syndrome according to both groups revealed similar differences, such as TLS-associated clinical symptoms and the urea levels at the onset of TLS and at the lowest GFR. Furthermore, there were significant changes in the phosphate level at the lowest GFR and the peak phosphate level.
The importance of daily serum phosphate level increase was revealed from statistical data performed according to the nephrological classification. A significant difference in nephrotoxic drug burden and patient numbers based on risk stratification assessment, which is an important determinant of TLS management, was found only in the “traditional” grouping (Table 1).
The daily change in serum phosphate level before the onset of AKI (− 3 to 0 days) proved to be the most significant discriminator for severe TLS–AKI, as demonstrated by ROC analysis (AUC = 0.727, p = 0.009). The cut-off value of daily change in serum phosphate concentration was 0.32 mmol/L. The ROC curve also shows that UA values varied in a wide range. The most accurate estimate found was the change in daily phosphate levels. A significant difference was revealed in two additional parameters, diuresis as measured in ml/kg/h and the peak of the phosphate level, with both weaker specificity and sensitivity compared to the change in daily phosphate (Fig. 2).
Evaluation of collected urine samples
According to the 24-h collected urinalysis samples, the direction of changes in urine and serum phosphate parameters, such as TMPO4/GFR, FE of phosphate, and serum phosphate levels were similar with an intensity depending on previous kidney injury and/or tumor cell turnover. In three cases, low GFR values (< 60 ml/min/1.73 m2) were observed in addition to elevated FENa, FE of phosphate (in two cases each), and TMPO4/GFR (in all three cases). All these parameters normalized later. Increased urinary Ca excretion was observed in two cases (Table 2).
Discussion
Regarding the literature, the relative incidence of TLS shows a considerable variation ranging between 3 and 30% of patients with leukemia and NHL; spontaneous TLS is more common in pediatric patients than in adults [1, 32, 33]. Roughly, 20–40% of all TLS cases have clinical manifestations (CTLS). Among pediatric patients, the relative incidence of TLS–AKI has been reported to be between 5 and 40% [32], and this rate can increase up to 75% in malignancies with large tumor burden [33]. So, the severe form of TLS mainly manifests as kidney injury [1]. The dialysis rate is 2–4% [33], and the TLS-related mortality is approximately 1.7% among high-risk patients [34].
In our retrospective study, the incidence of TLS was low (3.4%). However, the ratio of more severe forms of TLS: CTLS vs. LTLS was relatively high. Early initiation of proper TLS prophylaxis and conservative treatment might have contributed to the fact that only a small number of patients had LTLS. The two most common laboratory abnormalities detected in our patient cohort were hyperuricemia and hyperphosphatemia according to Cairo–Bishop criteria with good agreement of literature [16, 35, 36].
In a study with adult patients, UA was the most sensitive predictor in the LTLS group according to the ROC curve, and in addition to higher UA levels, kidney failure also occurred more often [9]. However, in our analysis, the change in UA level did not show a significant difference.
Interestingly, in many cases, hypophosphatemia preceded TLS. The presence of hypophosphatemia before the introduction of chemotherapy has been shown to be a significant risk factor for TLS [16]. It was proposed that its development is most often caused by tumor progression (increased phosphate utilization by malignant cells) in combination with reduced intake, primary tubular disorder, or acquired tubular cell dysfunction [37, 38]. Low extra- and consequent intracellular phosphate levels may cause impairment of tubular reabsorption due to low intracellular ATP formation [38, 39]. The increased excretion of phosphate and a consequentially increased risk of nephrocalcinosis raised the possibility that these pathological events may play an important role in the development of severe TLS and TLS–AKI [16]. It is notable that the evaluation of collected urine samples in our study did not confirm any significant impairment of tubular phosphate excretion in patients without AKI.
The reason for the increased phosphate level in TLS is still debated—the most plausible explanation is cell disruption and consequent kidney failure. The renal excretion of phosphate is highly efficient in patients with normal kidney function; acute hyperphosphatemia usually resolves within a few hours (6–12 h) [40]. A number of studies showed that serum phosphate increased only when there was a substantial reduction in GFR (GFR < 60 mL/min/1.73 m2) [40].
Previous studies have highlighted the importance of changes in phosphate levels as a marker of AKI progression [40] and an indicator of clinical outcomes in critically ill patients [41]. Darmon et al. examined the importance of phosphate levels from the point of view of TLS. In the TLS risk assessment score serum phosphate value is one of the factors which determine CTLS. A 1 mmol/L increase in serum phosphate level was associated with a fivefold increase in CTLS risk [42]. An adult TLS study by Lemerle et al. suggested that increases in serum phosphate levels appear to be a good predictive factor for AKI–TLS. The warning peak value of serum phosphate was 2.1 mmol/L [43].
Due to significant deviations from normal phosphate levels in childhood [28] and the frequent occurrence of initial hypophosphatemia, we focused on the daily changes in serum phosphate levels. Our findings showed a significant discriminatory capacity by ROC analysis for severe TLS–AKI, where the cut-off value was determined to be 0.32 mmol/L in the daily change in serum phosphate level.
Numerous studies have investigated the role of nephrotoxic drugs as contributory factors to kidney damage in subclinical kidney involvement [26, 44,45,46]. Our study also showed that patients with severe TLS received a substantial number of nephrotoxic drugs.
With the development of biomarker-guided risk assessment [47], the nomenclature of kidney failure has broadened, and the use of tubular markers enables earlier recognition of AKI, which is important not only for optimizing treatment, but also for preventing chronic complications. This is complemented by the alert systems—one of the best-known of which is the RAI (renal angina index) [48, 49]. RAI is based on the estimation of general risk and the clinical symptoms characterized by changes in creatinine and fluid overload. Monitoring for the latter is particularly important, since these patients are excessively hydrated. The risk of overfilling is high in case of kidney involvement. The automated RAI + tubular marker (e.g. NGAL (neutrophil gelatinase-associated lipocalin)) clinical decision support programs [50] and calculators used to monitor nephrotoxic drugs are becoming more and more popular to promote quick recognition of AKI. Implementation of monitoring the increase of daily phosphate levels into the RAI system may help in the management of TLS patients, enabling early recognition of high-risk patients.
Conclusion
Childhood TLS was retrospectively analyzed in our national study with a focus on nephrologic complications. Close monitoring of daily changes in the serum phosphate levels were shown to be an important factor for the recognition of severe TLS–AKI, as it can be considered a cost-effective laboratory marker of kidney involvement. A multidisciplinary approach is necessary to plan early preventive steps, such as optimization of hydration, application of adjuvant allopurinol and rUO treatment, and avoiding nephrotoxic drugs as much as possible. KRT remained an effective treatment modality of the most severe forms of TLS–AKI.
This could be the basis for further studies on the relationship between phosphate level and TLS. The major limitation of our study is the low number of patients, which may have a distorting effect on the results. Further studies with additional sensitive biomarkers and long-term follow-up data are needed for early detection and optimal management of severe TLS–AKI.
Data availability
Datasets analyzed during the study are patients’ data available in their medical documentation and the patients’ electronic database (MedSolution).
Abbreviations
- ALL:
-
Acute lymphoid leukemia
- AKI:
-
Acute kidney injury
- Ca:
-
Calcium
- Cr:
-
Creatinine
- CTLS:
-
Clinical tumor lysis syndrome
- FE:
-
Fractional excretion
- F-pRIFLE:
-
Failure category according to pRIFLe classification
- GFR:
-
Glomerular filtration rate
- HPOG:
-
Hungarian Pediatric Oncology Group
- I-pRIFLE:
-
Injury category according to pRIFLE classification
- K:
-
Potassium
- KRT:
-
Kidney replacement therapy
- LDH:
-
Lactate dehydrogenase
- LTLS:
-
Laboratory tumor lysis syndrome
- Na:
-
Sodium
- NGAL:
-
Neutrophil gelatinase-associated lipocalin
- R-pRIFLE:
-
Risk category according to pRIFLe classification
- RAI:
-
Renal angina index
- rUO:
-
Recombinant urate-oxidase
- se:
-
Serum
- TLS:
-
Tumor lysis syndrome
- TmPO4/GFR:
-
Ratio of maximum rate of renal tubular reabsorption of phosphate to GFR
- UA:
-
Uric acid
- u:
-
Urine
- WBC:
-
White blood cell
References
Saeed F, Ali MS, Ashraf MS, Vadsaria K, Siddiqui DE (2018) Tumour Lysis Syndrome in children with haematological cancers: experience at a tertiary care hospital in Karachi. J Pak Med Assoc 68:1625–1630
Howard SC, Jones DP, Pui C-H (2018) The tumor lysis syndrome. N Engl J Med 379:1094. https://doi.org/10.1056/NEJMra0904569
Cheung WL, Hon KL, Fung CM, Leung AK (2020) Tumor lysis syndrome in childhood malignancies. Drugs Context 9:1–14. https://doi.org/10.7573/dic.2019-8-2
Mika D, Ahmad S, Guruvayoorappan C (2012) Tumour lysis syndrome: implications for cancer therapy. Asian Pac J Cancer Prev 13:3555–3560. https://doi.org/10.7314/apjcp.2012.13.8.3555
Lupușoru G, Ailincăi I, Frățilă G, Ungureanu O, Andronesi A, Lupușoru M, Banu M, Văcăroiu I, Dina C, Sinescu I (2022) Tumor lysis syndrome: an endless challenge in onco-nephrology. Biomed 10:1012. https://doi.org/10.3390/biomedicines10051012
Shimada M, Johnson RJ, May WS Jr, Lingegowda V, Sood P, Nakagawa T, Van QC, Dass B, Ejaz AA (2009) A novel role for uric acid in acute kidney injury associated with tumour lysis syndrome. Nephrol Dial Transplant 24:2960–2964. https://doi.org/10.1093/ndt/gfp330.19581334
Lacatelli F, Rossi F (2005) Incidence and pathogenesis of tumor lysis syndrome. Contrib Nephrol 147:61–68. https://doi.org/10.1159/000082543
Strauss PZ, Hamlin SK, Dang J (2017) Tumor lysis syndrome: a unique solute disturbance. Nurs Clin North Am 52:309–320. https://doi.org/10.1016/j.cnur.2017.01.008
Ejaz AA, Pourafshar N, Mohandas R, Smallwood BA, Johnson RJ, Hsu JW (2015) Uric acid and the prediction models of tumor lysis syndrome in AML. PLoS One 10:0119497. https://doi.org/10.1371/journal.pone.0119497
Sanchez-Lozada LG, Tapia E, Santamaria J, Avila-Casado C, Soto V, Nepomuceno T (2005) Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int 67:237–247. https://doi.org/10.1111/j.1523-1755.2005.00074.x
Jones GL, Will A, Jackson GH, Webb NJ, Rule S (2015) Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br J Haematol 169:661–671. https://doi.org/10.1111/bjh.13403
Cairo MS, Coiffier B, Reiter A, Younes A (2010) Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol 149:578–586. https://doi.org/10.1111/j.1365-2141.2010.08143
Yulistiani TC, Ugrasena IDG, Qibtiyah M (2021) Hydration effect on kidney function and serum electrolyte in children with tumor lysis syndrome (TLS) and risk of TLS. J Basic Clin Physiol Pharmacol 32:603–609. https://doi.org/10.1515/jbcpp-2020-0412
Alakel N, Middeke JM, Schetelig J, Bornhäuser M (2017) Prevention and treatment of tumor lysis syndrome, and the efficacy and role of rasburicase. Onco Targets Ther 10:597–605. https://doi.org/10.2147/OTT.S103864
Personett HA, Barreto EF, McCullough KB, Dierkhising R, Leung N, Habermann TM (2019) Impact of early rasburicase on incidence of clinical tumor lysis syndrome in lymphoma. Leuk Lymphoma 60:2271–2277. https://doi.org/10.1080/10428194.2019.1574000
Ahn YH, Kang HJ, Shin HY, Ahn HS, Choi Y, Kang HG (2011) Tumour lysis syndrome in children: experience of last decade. Hematol 29:196–201. https://doi.org/10.1002/hon.995
Martens KL, Khalighi PR, Li S, White AA, Silgard E, Frieze D, Estey E, Garcia DA, Hingorani S, Li A (2020) Comparative effectiveness of rasburicase versus allopurinol for cancer patients with renal dysfunction and hyperuricemia. Leuk Res 89:106298. https://doi.org/10.1016/j.leukres.2020.106298
Shaikh SA, Marini BL, Hough SM, Perissinotti AJ (2018) Rational use of rasburicase for the treatment and management of tumor lysis syndrome. J Oncol Pharm Pract 24:176–184. https://doi.org/10.1177/1078155216687152
Savva DA, Herrera N, Rohatgi R (2018) Comparison of fixed versus traditional weight-based dosing of rasburicase in a pediatric population. Pediatr Blood Cancer 65:e27236. https://doi.org/10.1002/pbc.27236
Raina R, Joshi H, Chakraborty R (2021) Changing the terminology from kidney replacement therapy to kidney support therapy. Ther Apher Dial 25:437–457. https://doi.org/10.1111/1744-9987.13584
Stanski NL, Fuhrman D, Basu RK (2021) Controversies in paediatric acute kidney injury and continuous renal replacement therapy: can paediatric care lead the way to precision acute kidney injury medicine? Curr Opin Crit Care 27:604–610. https://doi.org/10.1097/MCC.0000000000000888
Matuszkiewicz-Rowinska J, Malyszko J (2020) Prevention and treatment of tumor lysis syndrome in the era of onco-nephrology progress. Kidney Blood Press Res 45:645–660. https://doi.org/10.1159/000509934
Zhang L, Chen D, Tang X, Li P, Zhang Y, Tao Y (2020) Timing of initiation of renal replacement therapy in acute kidney injury: an updated meta-analysis of randomized controlled trials. Ren Fail 42:77–88. https://doi.org/10.1080/0886022X.2019.1705337
Canet E, Zafrani L, Lambert J, Thieblemont C, Galicier L, Schnell D et al (2013) Acute kidney injury in patients with newly diagnosed high-grade hematological malignancies impact on remission and survival. PLoS One 8:55870. https://doi.org/10.1371/journal.pone.0055870
Ehrmann S, Helms J, Joret A, Martin-Lefevre L, Quenot JP, Herbrecht JE et al (2019) Nephrotoxic drug burden among 1001 critically ill patients: impact on acute kidney injury Clinical research in intensive care and sepsis-Trial group for global evaluation and research in sepsis (CRICS-TRIGGERSEP network). Ann Intensive Care 9:106. https://doi.org/10.1186/s13613-019-0580-1
Gobel BH (2002) Management of tumor lysis syndrome: prevention and treatment. Semin Oncol Nurs 18(3 Suppl 3):12–16. https://doi.org/10.1016/s0749-2081(02)80101-2
Sutherland SM, Byrnes JJ, Kothari M, Longhurst CA, Dutta S, Garcia P, Goldstein SL (2015) AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol 10:554–561. https://doi.org/10.2215/CJN.01900214
KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 Update. Recommendation 7: Bone mineral and vitamin d requirements and therapy. https://kidneyfoundation.cachefly.net/professionals/KDOQI/guidelines_ped_ckd/cpr7.htm. Accessed 9 March 2023
Matos V, van Melle G, Boulat O, Markert M, Bachmann C, Guignard JP (1997) Urinary phosphate/creatinine, calcium/creatinine, and magnesium/creatinine ratios in a healthy pediatric population. J Pediatr 131:252–257. https://doi.org/10.1016/s0022-3476(97)70162-8
Sargent JD, Stukel TA, Kresel J, Klein RZ (1993) Normal values for random urinary calcium to creatinine ratios in infancy. J Pediatr 123:393–397. https://doi.org/10.1016/s0022-3476(05)81738-x
Vallon V (2016) Tubular transport in acute kidney injury: relevance for diagnosis, prognosis and intervention. Nephron 134:160–166. https://doi.org/10.1159/000446448
Williams SM, Killeen AA (2017) Tumor lysis syndrome. Arch Pathol Lab Med 143:386–393. https://doi.org/10.5858/arpa.2017-0278-RS
Naeem B, Moorani KN, Anjum M, Imam U (2019) Tumor lysis syndrome in pediatric acute lymphoblastic leukemia at tertiary care center. Pak J Med Sci 35:899–904. https://doi.org/10.12669/pjms.35.4.715
Gopakumar KG, Seetharam S, Km JK, Nair M, Rajeswari B, Cs G, Vr P, Thankamony P (2018) Risk-based management strategy and outcomes of tumor lysis syndrome in children with leukemia/lymphoma: Analysis from a resource-limited setting. Pediatr Blood Cancer 65:e27401. https://doi.org/10.1002/pbc.27401
El-Husseini A, Sabucedo A, Lamarche J, Courville C, Peguero A (2012) Acute kidney injury associated with tumor lysis syndrome: a paradigm shift. Am J Emerg Med 30:390.e3–6. https://doi.org/10.1016/j.ajem.2010.12.029
Xue Y, Chen J, Gao S, Zhai X, Wang N, Gao J et al (2021) Clinical characteristics of tumor lysis syndrome in childhood acute lymphoblastic leukemia. Sci Rep 11:9656. https://doi.org/10.1038/s41598-021-88912-2
Miltiadous G, Christidis D, Kalogirou M, Elisaf M (2008) Causes and mechanisms of acid-base and electrolyte abnormalities in cancer patients. Eur J Intern Med 19:1–7. https://doi.org/10.1016/j.ejim.2007.04.016
Liamis G, Elisaf M (2000) Hypokalemia, hypophosphatemia and hypouricemia due to proximal renal tubular dysfunction in acute myeloid leukemia. Eur J Haematol 64:277–278. https://doi.org/10.1034/j.1600-0609.2000.9l123.x
Coule S, Masters DW, Barnard D (1992) TmP/GFR and ionized calcium in the management of severe hypophosphatemia. Ann Clin Biochem 29:567–569. https://doi.org/10.1177/000456329202900516
Carfagna F, Del Vecchio L, Pontoriero G, Locatelli F (2018) Current and potential treatment options for hyperphosphatemia. Expert Opin Drug Saf 17:597–607. https://doi.org/10.1080/14740338.2018.1476487
Zheng WH, Yao Y, Zhou H, Xu Y, Huang HB (2022) Hyperphosphatemia and outcomes in critically ill patients: a systematic review and meta-analysis. Front Med 9:870637. https://doi.org/10.3389/fmed.2022.870637
Darmon M, Vincent F, Camous L, Canet E, Bonmati C, Braun T, Caillot D et al (2013) Tumour lysis syndrome and acute kidney injury in high-risk haematology patients in the rasburicase era. A prospective multicentre study from the Groupe de Recherche en Réanimation Respiratoire et Onco-Hématologique. Br J Haematol 162:489–497. https://doi.org/10.1111/bjh.12415
Lemerle M, Schmidt A, Thepot-Seegers V, Kouatchet A, Moal V, Raimbault M, Orvain C et al (2022) Serum phosphate level and its kinetic as an early marker of acute kidney injury in tumor lysis syndrome. J Nephrol. https://doi.org/10.1007/s40620-022-01263-7
Malyszko J, Kozlowska K, Kozlowski L, Malyszko J (2017) Nephrotoxicity of anticancer treatment. Nephrol Dial Transplant 32:924–936. https://doi.org/10.1093/ndt/gfw338
Holsteen PE, Gist KM, Brinton JT, Hebert M, Iwanowski M, Kim A, Leath A, Shah A, Soranno DE, Marschner MN (2022) Nephrotoxic exposures and acute kidney injury in noncritically ill children stratified by service. Hosp Pediatr 12:866–877. https://doi.org/10.1542/hpeds.2021-006169
Goldstein SL, Dahale D, Kirkendall ES, Mottes T, Kaplan H, Muething S (2020) A prospective multi-center quality improvement initiative (NINJA) indicates a reduction in nephrotoxic acute kidney injury in hospitalized children. Kidney Int 97:580–588. https://doi.org/10.1016/j.kint.2019.10.015
Gist KM, Fuhrman D, Stanski N, Menon S, Soranno DE (2022) Subphenotypes of acute kidney injury in children. Curr Opin Crit Care 28:590–598. https://doi.org/10.1097/MCC.0000000000000986
Stanski NL, Krallman KA, Chima RS, Goldstein SL (2022) A risk-stratified assessment of biomarker-based acute kidney injury phenotypes in children. Pediatr Res. https://doi.org/10.1038/s41390-022-02233-2
Stanski NL, Wong HR, Basu RK, Cvijanovich NZ, Fitzgerald JC, Weiss SL, Bigham MT, Jain PN, Schwarz A, Lutfi R, Nowak J, Allen GL, Thomas NJ, Grunwell JR, Quasney M, Haileselassie B, Chawla LS, Goldstein SL (2021) Recalibration of the renal angina index for pediatric septic shock. Kidney Int Rep 6:1858–1867. https://doi.org/10.1016/j.ekir.2021.04.022
Goldstein SL, Krallman KA, Kirby C, Roy JP, Collins M, Fox K, Schmerge A, Wilder S, Gerhardt B, Chima R, Basu RK, Chawla L, Fei L (2022) Integration of the renal angina index and urine neutrophil gelatinase-associated lipocalin improves severe acute kidney injury prediction in critically ill children and young adults. Kidney Int Rep 7:1842–1849. https://doi.org/10.1016/j.ekir.2022.05.021
Acknowledgements
The authors are grateful to Zsuzsanna Jakab for providing data from the Hungarian Pediatric Tumor Registry.
Funding
Open access funding provided by University of Debrecen.
Author information
Authors and Affiliations
Contributions
All of the listed authors contributed significantly to the publication. National data collection was done at the following centers: DE, GK (Budapest), MS, KB (Szeged), GO (Pécs) and PV, IS, CK (Debrecen). The study was conceived and designed by EB, CK, and TS. FV helped in the selection of statistical methods and the implementation of the statistical analysis. EB analyzed and interpreted the data of pediatric patients with hemato-oncological diseases. TS and CK were major contributors in writing the manuscript. CK and TS contributed equally to this manuscript and shared senior authorship. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Scientific Research Ethical Committee of the Medical Research Council of Hungary (ETT TUKEB IV/8068–1/2020/EKU) and was performed according to the 2008 Declaration of Helsinki.
Consent for publication
The authors give consent to publish this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Biró, E., Erdélyi, D., Varga, P. et al. Daily serum phosphate increase as early and reliable indicator of kidney injury in children with leukemia and lymphoma developing tumor lysis syndrome. Pediatr Nephrol 38, 3117–3127 (2023). https://doi.org/10.1007/s00467-023-05923-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-05923-z