Abstract
Background
Technological advancements in the operating room (OR) have sparked new challenges for surgical workflow, OR professionals, and patient safety. Disruptive events are frequent across all surgical specialties, but little is known about their effects on patient outcomes and the influence of systemic factors. The aim was to explore the associations of intraoperative flow disruptions (FDs) with patient outcomes, staff workload, and surgery duration.
Methods
Prospective, single-center, and multi-source study comprising direct and standardized OR observations of urologic surgical procedures, clinical patient outcomes, and staff- and patient-reported outcome data (PROMs; 3-month follow-up). All data were recorded between 01/2020 and 10/2021. FDs were assessed using standardized procedure observations. Linear and logistic regression analyses including multiple system factors were used to explore the effects of FDs on surgical outcomes.
Results
61 robotic-assisted radical prostatectomy procedures were captured (with 61 patients and 243 staff reports). High rates of FDs were observed; however, our analyses did not show significant relationships with patient complication rates. Equipment- and patient-related FDs were associated with increased staff workload. No association was found between higher rates of FDs and procedure duration.
Conclusions
FDs were not related to inferior patient outcomes. Our findings may inform future OR investigations that scrutinize the complex interplay of human, team, process, and technological components that mitigate the effects of FDs during surgery.
Graphical abstract

Similar content being viewed by others
Advancements in the field of surgical technology have been remarkable over the past few years. Robotic systems in the operating room (OR) have been widely implemented to improve workplace ergonomics and patient care [1].
Recently, there has been growing interest in the effects of flow disruptions (FDs) in the OR on the workload of surgical teams and patient safety [2]. Since FDs, such as telephone calls or equipment failures, occur frequently and potentially increase teams’ workload, they pose an inherent risk [3]. The increasing application of technology creates new opportunities for FDs (i.e., technical errors). Several studies have shown that stress and workload levels of OR team members increase as a consequence of frequent FDs [4]. In particular, FDs caused by technical devices can cause adverse effects and significant delays [5, 6]. However, findings on the impact of FDs in surgical work are heterogeneous, and it can be assumed that the effects depend on several factors such as task complexity, nature of FDs, and quality of teamwork [7].
Although, FDs’ consequences for patient safety should be in the focus, the current literature is small, inconsistent, and based mainly on simulation studies [8, 9]. We aim to respond to the call for more comprehensive approaches by combining the traditional human factors perspective with patient-centered research and patient-reported outcomes [10]. We assumed a dynamic relationship between FDs and patient outcomes depending on multiple factors, the timing of FDs, and the individual nature (i.e., cause type).
Materials and methods
Study design and setting
An observational cohort study utilizing a mixed-methods design was applied: We combined intraoperative expert observations with staff self-reports, patient data from hospital records, and patient survey follow-up data.
The investigation was conducted in the urological department of a university hospital in southern Germany. All patients underwent radical prostatectomies and were operated with a da Vinci® surgical robotic system (Models Si and X, Intuitive Inc., Sunnyvale CA). The data collection took place between January 2020 and June 2021. The follow-up period ended four months later (October 2021). Ethical approval was obtained from the local ethics board (reference number 19–696). The study protocol was registered at clinicaltrials.gov (ID: NCT04226391). The dataset generated during this analysis is available online on the OSF platform (https://osf.io/tqe42/). The STROBE guidelines for reporting of observational studies were followed [11]. Due to the limited evidence base, we were unable to estimate the required sample size for the association between FDs and patient outcomes.
Study procedure
Before the start of the data collection, pilot observations (~ 200 h in total) were conducted to train observers, minimize Hawthorne bias, and finalize data collection tools. We closely collaborated with surgical staff members to ensure that observers were familiar with the procedure and required surgical steps. Furthermore, this allowed local surgical teams to familiarize themselves with the presence of external observers. We also determined inter-rater agreement for the observational tools.
During the main study period, all elective patients listed for robotic-assisted radical prostatectomy were considered potentially eligible if a trained observer was available. Exclusion criteria were as follows: (1) patient absent for obtaining informed consent (on day before procedure), (2) refusal to participate in the study, (3) patient lacks language skills (German/English) for informed consent, (4) surgery canceled/rescheduled at short notice, (5) observer not available at short notice, and (6) surgical intervention canceled or substantially changed intraoperatively.
On the day before surgery, patients were informed about study purpose and procedure by a study member, and written consent was obtained. OR staff were informed about the study in regular meetings. Before the start of each data collection, all OR team members were asked if they were aware of the study and agreed with observer presence.
Perioperative observations were conducted by one observer and data were recorded on a standardized worksheet. We divided each procedure into three consecutive phases: (1) pre-console (first incision to console start), (2) console time, and (3) post-console (undocking to final suture) [12].
After each surgery, staff members were asked to answer a short questionnaire. All OR staff members being present during the surveyed procedure and who had completed their professional education were eligible to participate. Written consent from participating staff members was obtained.
Measures
Patient and surgery characteristics
Patient age (in years), body mass index (BMI), American Society of Anesthesiology (ASA) score, prostate-specific antigen (PSA) levels, and Gleason grading score for prostate cancer [13] were obtained from the hospital records. The number of active staff members, staff, and console changes were recorded. Staff members rated ‘team familiarity’ based on the number of surgeries performed together (last six months) [14].
Flow disruptions
We applied a common definition of FDs as events ‘that potentially distract staff members from their primary tasks or cause a break in task execution’ [15]. This included unanticipated minor and major events such as small talk, equipment failures and coordination problems, and excluded planned interruptions such as the WHO-checklist timeout. In line with previous studies [12, 15], FD cause categories were defined as ‘external factors,’ ‘communication,’ ‘equipment,’ ‘coordination,’ ‘training/teaching,’ ‘patient factors,’ ‘surgeon task considerations,’ and ‘environmental factors.’ Table 1 shows definitions and examples for each FD category [16].
Severity of each FD was rated on a three-point scale: (0) potential impact; (1) clear impact (i.e., task break); (2) high risk for patient safety (e.g., defective aspirator while bleeding). Interrater agreement (IRA) was calculated using Gwet’s AC2 coefficient[17]. We obtained an IRA of Gwet’s AC2 of 0.92 for the cause categories of FDs and of 0.89 for FD severity ratings.
Outcome measures
Patient outcomes: Clinical outcomes were combined with patient-reported outcome measures (PROMs). From hospital records, we retrieved data on complication rates (e.g., surgical site infections), including readmission to hospital, duration of hospital and intensive care unit (ICU) stay, and patients’ pre- and postoperative blood parameters as indicators of inflammation (C-reactive protein and leukocyte count). Postoperative complications were graded by an experienced urologist using the Clavien-Dindo classification system [18]. The day before surgery, each patient was asked to fill out a questionnaire on erectile function, incontinence status, and current quality of life (PROMs). The International Index of Erectile Function (five-item version, IIEF-5) [19] and International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-UI SF) [20] were used. For IIEF, an overall score was calculated with a possible range of 0–25 (a higher score indicates better function). The ICIQ overall score is interpreted as 'no incontinence' (0 points), 'mild incontinence' (1–5 points), 'moderate incontinence' (6–10 points), and 'strong incontinence' (> 10 points). In addition, we used 28 items of the established EORTC QLQ-C30 instrument, which was designed to assess the quality of life of cancer patients [21]. Its 28 items consist of questions about symptoms and functioning (e.g., ‘Did you need to rest?’) and are rated on a four-point scale, with a lower score indicating a better quality of life. We calculated an overall score that was then included in our further analysis. Three months after surgery, each patient received the same questionnaire via mail. We defined complication rates within the first 30 days after surgery as the primary outcome. Baseline measures for outcomes with pre- and post-surgery differences (Δ) used the day before surgery as baseline. Blood parameter follow-ups were taken on the first postoperative day, and PROM follow-ups were collected three months post-surgery. It should be noted that both, continence and erectile function, recover after prostate removal gradually over a long period (> 12 months) [22]. The reported outcomes do presumably not reflect the final endpoints of functional recovery and should not be interpreted as ultimate outcomes of RAS radical prostatectomies. Nevertheless, for our analysis, exclusively the differences in recovery between patients is relevant, and therefore, the application of the 3-months endpoint is suitable [22]. A summary of included patient outcomes can be found in Supplement 1 (eTable 1).
Staff outcomes: To assess the intraoperative workload level of OR staff three items from the Surgery Task Load Index (SURG-TLX) [23], an adapted version of the NASA Task Load Index, were used [24]. Items ‘situational stress,’ ‘time pressure,’ and ‘complexity’ had to be rated on a continuous scale from 0 to 100 (100 = maximum task load). We combined the overall workload level scores of individual staff members into a joint overall team score.
Procedural outcomes: Surgery duration (in minutes) and individual surgical phase duration were recorded, respectively.
Statistical analyses
Descriptive statistics (means, standard deviations) were calculated for continuous data. Repeated measures analysis of variance (ANOVA) was applied to check for significant changes in FD rates between surgical phases. Pearson correlation analyses have been used to determine the relationship of FD categories among each other. To assess the relationship between FDs and patient, provider, and procedure outcomes, linear and logistic regression analyses were applied. Patient and procedure characteristics were considered as covariates and relevant predictors were included in the adjusted regression models. All not normally distributed metric outcome variables were transformed using natural logarithm (i.e., ‘length of hospital stay,’ ‘erectile function,’ ‘workload surgeons’). Non-standardized (B) and standardized (ß) regression coefficients are reported. To consider the potentially confounding influence of surgeons' technical performance [25], we assessed the relationship between surgeons' workload and patient outcomes. As additional analyses, we tested whether the relationship between FDs and outcomes changed between different surgical phases. We applied a p-level of 0.05 for all statistical analyses. To address the problem of multiple comparisons, we conducted a Bonferroni correction with a corrected p-level of padjusted = 0.05/27 = 0.0019 for our main analysis. All data were entered and processed using SPSS Statistics version 27 (IBM Corp., Armonk, NY). Authors MW and AK were responsible for the statistical analyses.
Results
Sample
61 elective robotic-assisted radical prostatectomy cases were included with a mean ‘skin-to-skin’ duration of 191.87 min (SD = 27.82). Of 93 potentially eligible patients, 32 were excluded. Figure 1 depicts procedures of patient and staff member inclusion. Patients (all male) had a mean age of 66.52 years (SD = 7.55), average BMI of 26.53 (SD = 4.38), mean PSA level of 15.77 ng/ml (SD = 19.32), and median Gleason score of 7b (min = 6, max = 9). ASA score was class 1 for 3.3% of included patients, class 2 for 42.6%, and class 3 for 54.1%.
Of 343 potentially eligible staff members, 125 surgeons, 75 nurses, and 43 anesthesiologists completed the postoperative questionnaire. Post-surgical time constraints were the most common reason for not answering the questionnaire.
A mean of 5.62 (SD = 0.80) staff members were present in surveyed procedures. On average 3.74 (SD = 1.86) intraoperative staff changes were recorded and a mean of 1.33 (SD = 1.72) console changes per procedure.
Intraoperative flow disruptions
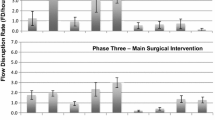
Overall, 4027 FDs were observed, with a mean of 66.02 FDs per surgery (SD = 17.24). The mean overall rate per hour was 20.52 (SD = 3.93). Total counts of observed FDs and descriptive statistics for each surgical phase are shown in Table 2.
Severity of FDs was evaluated as ‘potential impact’ for 3669 FDs (91.1%), ‘clear impact’ for 349 (8.7%), and ‘high risk’ for 3 FDs (0.1%). Six FDs were excluded because of incomplete data.
Repeated measures ANOVA (df = 2; F = 16.99) revealed a significant drop in overall FD rates from surgical Phase 1 to 2 (p < 0.001), but no significant difference from Phase 2 to 3 (p ~ 1.00).
We found a significant negative correlation between external FDs and coordination-related FDs (r = -0.31; p = 0.017). Patient FDs were related to equipment-related FDs (r = 0.29; p = 0.022) and negatively associated to teaching- and training-related FDs (r = -0.31; p = 0.015). All correlations between the FD source categories can be found in the Supplement 2 (eTable2).
Patient, staff, and procedure outcomes
Descriptive statistics of all relevant endpoints can be found in Table 3.
In two procedures, intraoperative complications were identified (ureter injury n = 1; respiratory failure n = 1) and in five patients’ postoperative complications (lymphocele n = 4, Clavien–Dindo Grade 3a; revision surgery needed n = 1, Clavien–Dindo Grade 3b). One patient was readmitted to the hospital within 30 days after surgery. The mean duration of hospital stay was 10.03 days (range: 8 to 20 days). Two patients spent two days postoperatively in the ICU for monitoring.
Inflammation indicators (CRP and leucocyte count) increased on average on the first postsurgical day. Patient-reported outcomes (PROMs) showed a decrease in erectile function and continence three months after surgical intervention compared to the preoperative baseline measurements (Table 3). Quality of life scores increased on average, indicating a deterioration of symptoms, and functioning in patients’ daily life.
Prospective associations of FD events on patient, staff, and procedure outcomes
No significant predictors (i.e., patient and procedure characteristics) were identified for primary patient outcomes (Supplement 2, eTable 3). Table 4 and Table 5 show the results of our main analyses. The regression models revealed no significant relationship between FDs and primary patient endpoints.
In our further analyses, we identified significant relationships between communication-related FDs (B = -1.01, ß = -0.27, p = 0.037) and training-related FDs (B = 0.29, ß = 0.30, p = 0.020) with changes in leukocyte counts, coordination FDs with change in incontinence (B = 3.05, ß = 0.40, p = 0.011), overall FD rates (B = -1.32, ß = -0.42, p = 0.014), and external FDs (B = -1.25, ß = 0.40, p = 0.023) with a change in the PROM quality-of-life scores.
Equipment-related FDs were significantly related to teams’ (B = 4.41, ß = 0.40, p < 0.001) and surgeons’ workload (ß = 0.34, p = 0.003). Likewise, a significant relationship between patient-related FDs and teams’ (B = 7.19, ß = 0.29, p = 0.010) and surgeons’ workload (ß = 0.30, p = 0.010) was found. Surgery duration was not associated with FDs.
The results of all univariate and adjusted regression analyses for primary and secondary endpoints can be found in Supplement 2 (eTable 4, eTable 5).
Surgeons’ workload and patient outcomes
There was a negative relationship between surgeons’ workload and length of hospital stay (ß = -0.27; p = 0.033). In addition, we found a relationship between surgeons’ workload with increased CRP (ß = 0.27; p = 0.030).
Effects of FDs per intraoperative phase
We identified a significant relationship between FD rates in Phase 2 and increased leukocyte counts (B = 0.12, ß = 0.27, p = 0.041). FDs during Phase 3 were associated with decreased leukocyte counts (B = -0.11, ß = -0.40, p = 0.002). In addition, we detected a significant relationship between a decrease in patients’ quality of life and FDs during Phase 2 (B = -1.08, ß = -0.39, p = 0.025). During Phases 1 and 2, the association between the count of FDs and phase duration remained significant, but this did not apply to Phase 3. Detailed results of this additional analysis can be found in the Supplement 2 (eTable 4).
Discussion
Understanding the complexity of the dynamic OR working system with its interactions of humans and technology is essential to safeguard the quality of surgical care. This is the first real-world OR investigation that comprehensively assessed FDs and key patient, staff, and procedural outcomes. In line with previous studies, we found FDs to be highly frequent in robotic-assisted surgeries (RAS) [26]. We did not find an association between FDs and primary patient outcomes. Still, our data suggest that specific causes of FDs are related to some of the secondary outcomes.
We did not identify a linear relationship between FDs and patient outcomes [2, 27]. We presume surgical teams to develop effective strategies to cope with prevalent FDs. Resilience research suggests that OR teams acquire and apply strategies for management of FD events (i.e., reducing FDs in high-risk situations) [28]. In particular, events caused by the OR team members themselves (i.e., small talk, refilling supplies) might be postponed to opportune moments [29]. Nevertheless, we cannot preclude that with an accumulation of adverse conditions in the dynamic OR system, major FDs may trigger adverse consequences for patient care, staff, or procedural outcomes [30].
Reported workload of surgeons and overall staff were moderate. A higher workload reported by surgeons was associated with a deterioration in two patient outcomes: length of hospital stays and CRP levels. This may indicate an impairment in surgical performance when workload is high [31]. Especially equipment- and device-related FDs were related to higher workload levels suggesting that the increasing use of technology in ORs indeed creates new challenges and novel demands for the OR team [32].
Lastly, our data did not show a significant association between FD rate and surgery duration. Previous studies suggested that FDs cause a significant extension of surgery duration [33, 34]. We propose potential post-hoc explanations: First, observed surgeries frequently included surgical training, which per se increases duration. Second, we did not record FD duration. Thus, the impact of individual, yet, long-lasting FDs might not be accounted for. Third, our study captured a large number of FDs without a break in main task activity (i.e., small talk and visitors). It is conceivable that these minor FDs do not substantially extend surgery time.
Limitations
Our findings should be interpreted in light of the following limitations:
First, we focused on one type of urological procedure to ensure better comparability of patient outcomes. Robotic-assisted radical prostatectomies result in a relatively low rate of complications. Therefore, external validity and generalizability should be cautiously considered. Our findings should be verified in a sample of more diverse surgical procedures including interventions with increased task and coordination complexities, high-risk procedures, and across various surgical specialties. Second, our choice of patient outcomes potentially limits the internal validity of our findings, although the selected measures are commonly applied to evaluate the success of a RAS radical prostatectomy, we might have missed further relevant patient outcomes, such as pain and tumor remission. Third, we explored associations (i.e., correlations) between FDs and our outcome measures. Intervention studies are necessary to determine causality. Fourth, since the required sample size for our main analysis couldn’t be estimated in advance, statistical power might be limited.
There were also some minor limitations: Our observations were made in a busy hospital environment, and it is conceivable that some FDs were missed. We minimized this risk through systematic training and ongoing reliability tests. We based our observations on a specific definition of FDs that has been applied in similar studies. Nevertheless, our methodology may not include all interruption events that have been identified as FDs by other authors. This also applies to our evaluation of potential impact of FDs events and the included high amount of minor events [4, 35]. Observers had a non-surgical background, what may limit their inferences concerning potential impact of FDs for surgical task complexity and natural progression of the surgical flow. During our data collection period, the local DaVinci model used was exchanged for a newer version after six months. To avoid including FDs related to the familiarization phase with the new model, we paused data collection for 10 weeks. Moreover, the study was impacted by the COVID-19 pandemic, yet all key steps in data collection were upheld.
Implications
Establishing a smooth surgical workflow and OR teamwork safeguard quality and safety of surgical care. However, in line with previous propositions, we deem the concept of a ‘sterile cockpit’ not fully applicable in the OR [36]. Safety improvements can be made through effective OR management (i.e., providing sufficient time for preparation), professional training (i.e., how to prevent and mitigate stressful situations, improving teamwork) [37], and thorough maintenance of technical equipment. We strongly believe that it is important to consider all components of OR work systems for effective interventions [38].
Future research should focus on investigating FDs’ role in the dynamic working system, and successful FD management strategies [39]. Influencing factors such as timing, teamwork, the individual nature of FDs, and FD interaction (i.e., cascade events) should be in focus of future research. Studies that comprehensively address multiple dimensions of OR work and consider existing strategies to deal with FDs could further improve the current study base [40]. Our findings should be verified in high-risk procedures and in larger or more heterogeneous patient samples.
Conclusion
This study was an in-depth investigation of the implications of intraoperative FDs for patients and surgical work using a system-oriented approach. Our data revealed that although the OR team experienced high rates of equipment- and patient-related FDs and significant workload levels, we did not find direct effects on primary patient outcomes. This suggests a degree of resilience against FDs, but we cannot preclude the possibility of adverse effects of (major) FDs in certain situations. Given the plethora of descriptive studies on FDs, we followed the call for more comprehensive research by accounting for relevant system factors. To further advance our knowledge, future research should seek to alleviate the negative consequences of major FDs and further elucidate the interplay of surgical workflow and contributing system factors.
References
Dalager T, Jensen PT, Eriksen JR et al (2020) Surgeons’ posture and muscle strain during laparoscopic and robotic surgery. Br J Surg 107:756–766. https://doi.org/10.1002/bjs.11394
Wiegmann DA, Sundt TM (2019) Workflow disruptions and surgical performance: past, present and future. BMJ Qual Saf. https://doi.org/10.1136/bmjqs-2018-008670
Rivera-Rodriguez AJ, Karsh B-T (2010) Interruptions and distractions in healthcare: review and reappraisal. Qual Saf Health Care 19:304–312. https://doi.org/10.1136/qshc.2009.033282
Koch A, Burns J, Catchpole K et al (2020) Associations of workflow disruptions in the operating room with surgical outcomes: a systematic review and narrative synthesis. BMJ Qual Saf 29:1033–1045. https://doi.org/10.1136/bmjqs-2019-010639
Sharma S, Grantcharov T, Jung JJ (2021) Non-technical skills and device-related interruptions in minimally invasive surgery. Surg Endosc 35:4494–4500. https://doi.org/10.1007/s00464-020-07962-1
Lear R, Riga C, Godfrey AD et al (2016) Multicentre observational study of surgical system failures in aortic procedures and their effect on patient outcomes. Br J Surg 103:1467–1475. https://doi.org/10.1002/bjs.10275
McMullan RD, Urwin R, Gates P et al (2021) Are operating room distractions, interruptions, and disruptions associated with performance and patient safety? A systematic review and meta-analysis. Int J Qual Health Care. https://doi.org/10.1093/intqhc/mzab068
Schraagen JM, Schouten T, Smit M et al (2011) A prospective study of paediatric cardiac surgical microsystems: assessing the relationships between non-routine events, teamwork and patient outcomes. BMJ Qual Saf 20:599–603. https://doi.org/10.1136/bmjqs.2010.048983
Yoong W, Khin A, Ramlal N et al (2015) Interruptions and distractions in the gynaecological operating theatre: irritating or dangerous? Ergonomics 58:1314–1319. https://doi.org/10.1080/00140139.2015.1005171
de Leval MR, Carthey J, Wright DJ et al (2000) Human factors and cardiac surgery: a multicenter study. J Thorac Cardiovasc Surg 119:661–672. https://doi.org/10.1016/S0022-5223(00)70006-7
Vandenbroucke JP, von Elm E, Altman DG et al (2014) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg 12:1500–1524. https://doi.org/10.1016/j.ijsu.2014.07.014
Catchpole K, Perkins C, Bresee C et al (2016) Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc 30:3749–3761. https://doi.org/10.1007/s00464-015-4671-2
Epstein JI (2010) An update of the Gleason grading system. J Urol 183:433–440. https://doi.org/10.1016/j.juro.2009.10.046
Xu R, Carty MJ, Orgill DP et al (2013) The teaming curve: a longitudinal study of the influence of surgical team familiarity on operative time. Ann Surg 258:953–957. https://doi.org/10.1097/SLA.0b013e3182864ffe
Mentis HM, Chellali A, Manser K et al (2016) A systematic review of the effect of distraction on surgeon performance: directions for operating room policy and surgical training. Surg Endosc 30:1713–1724. https://doi.org/10.1007/s00464-015-4443-z
Souders CP, Catchpole K, Hannemann A et al (2019) Flow disruptions in robotic-assisted abdominal sacrocolpopexy: does robotic surgery introduce unforeseen challenges for gynecologic surgeons? Int Urogynecol J 30:2177–2182. https://doi.org/10.1007/s00192-019-03929-6
Tran D, Dolgun A, Demirhan H (2020) Weighted inter-rater agreement measures for ordinal outcomes. Commun Stat Simul Comput 49:989–1003. https://doi.org/10.1080/03610918.2018.1490428
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Rosen RC, Cappelleri JC, Smith MD et al (1999) Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 11:319–326. https://doi.org/10.1038/sj.ijir.3900472
Avery K, Donovan J, Peters TJ et al (2004) ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn 23:322–330. https://doi.org/10.1002/nau.20041
Fayers P, Bottomley A (2002) Quality of life research within the EORTC—the EORTC QLQ-C30. Eur J Cancer 38:125–133
Kretschmer A, Bischoff R, Chaloupka M et al (2020) Health-related quality of life after open and robot-assisted radical prostatectomy in low- and intermediate-risk prostate cancer patients: a propensity score-matched analysis. World J Urol 38:3075–3083. https://doi.org/10.1007/s00345-020-03144-9
Wilson MR, Poolton JM, Malhotra N et al (2011) Development and validation of a surgical workload measure: the surgery task load index (SURG-TLX). World J Surg 35:1961–1969. https://doi.org/10.1007/s00268-011-1141-4
Lowndes BR, Forsyth KL, Blocker RC et al (2020) NASA-TLX assessment of surgeon workload variation across specialties. Ann Surg 271:686–692. https://doi.org/10.1097/SLA.0000000000003058
Haglind E, Carlsson S, Stranne J et al (2015) Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: a prospective, controlled, nonrandomised trial. Eur Urol 68:216–225. https://doi.org/10.1016/j.eururo.2015.02.029
Jung JJ, Jüni P, Lebovic G et al (2018) First-year analysis of the operating room black box study. Ann Surg 271:122–127. https://doi.org/10.1097/SLA.0000000000002863
Joseph A, Khoshkenar A, Taaffe KM et al (2018) Minor flow disruptions, traffic-related factors and their effect on major flow disruptions in the operating room. BMJ Qual Saf. https://doi.org/10.1136/bmjqs-2018-007957
Jung JJ, Elfassy J, Grantcharov T (2019) Factors associated with surgeon’s perception of distraction in the operating room. Surg Endosc. https://doi.org/10.1007/s00464-019-07088-z
Dreyfus D, Nair A (2022) The impact of operational disruptions on performance in surgical settings: moderating roles of risk management infrastructure and information exchange. IJOPM 42:930–958. https://doi.org/10.1108/IJOPM-08-2021-0524
Allers JC, Hussein AA, Ahmad N et al (2016) Evaluation and Impact of Workflow Interruptions during robot-assisted surgery. Urology 92:33–37. https://doi.org/10.1016/j.urology.2016.02.040
Arora S, Sevdalis N, Nestel D et al (2010) The impact of stress on surgical performance: a systematic review of the literature. Surgery 147(318–30):330.e1–6. https://doi.org/10.1016/j.surg.2009.10.007
Schreyer J, Koch A, Herlemann A et al (2021) RAS-NOTECHS: validity and reliability of a tool for measuring non-technical skills in robotic-assisted surgery settings. Surg Endosc. https://doi.org/10.1007/s00464-021-08474-2
Al-Hakim L (2011) The impact of preventable disruption on the operative time for minimally invasive surgery. Surg Endosc 25:3385–3392. https://doi.org/10.1007/s00464-011-1735-9
von StraussundTorney M, Dell-Kuster S, Hoffmann H et al (2016) Microcomplications in laparoscopic cholecystectomy: impact on duration of surgery and costs. Surg Endosc 30:2512–2522. https://doi.org/10.1007/s00464-015-4512-3
Al-Hakim L, Xiao J, Sengupta S (2017) Ergonomics perspective for identifying and reducing internal operative flow disruption for laparoscopic urological surgery. Surg Endosc 31:5043–5056. https://doi.org/10.1007/s00464-017-5568-z
Bruun B, Poulsen JL, Møhl P et al (2021) Is non-stop always better? Examining assumptions behind the concept of flow disruptions in studies of robot-assisted surgery. J Robot Surg 16:731–733. https://doi.org/10.1007/s11701-021-01275-8
van Houwelingen BCG, Rutkowski A-F, Ganni S et al (2019) Effects of surgical flow disruptions on surgeons’ resources: a pilot study. Surg Endosc. https://doi.org/10.1007/s00464-019-07239-2
Law KE, Hildebrand EA, Hawthorne HJ et al (2018) A pilot study of non-routine events in gynecological surgery: Type, impact, and effect. Gynecol Oncol 152:298–303. https://doi.org/10.1016/j.ygyno.2018.11.035
Ayas S, Armstrong B, Wong S et al (2022) Mitigating operating room distractions: a systematic review assessing intervention effectiveness. Hum Factors Healthcare 2:100013. https://doi.org/10.1016/j.hfh.2022.100013
Göras C, Olin K, Unbeck M et al (2019) Tasks, multitasking and interruptions among the surgical team in an operating room: a prospective observational study. BMJ Open 9:e026410. https://doi.org/10.1136/bmjopen-2018-026410
Acknowledgements
The authors would like to thank all participating patients and gratefully acknowledge the contributions of participating urologists, nurses, and anesthetists who supported this study despite the additional work demands and clinical challenges throughout the COVID-19 pandemic.
Funding
Open Access funding enabled and organized by Projekt DEAL. Amelie Koch is financially supported by the Munich Centre of Health Sciences (MC Health). Ken Catchpole is financially supported by Agency for Healthcare Research and Quality (Grant Number: R01 HS26491-01). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Matthias Weigl and Amelie Koch had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Munich Centre of Health Sciences (MC Health),Agency for Healthcare Research and Quality,R01 HS26491-01,Ken Catchpole
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Mrs. Amelie Koch, Dr. Caroline Quartucci, Prof. Dr. Alexander Buchner, Prof. Dr. Boris Schlenker, Prof. Dr. Armin Becker, Prof. Dr. Ken Catchpole, and Prof. Dr. Matthias Weigl have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koch, A., Quartucci, C., Buchner, A. et al. Associations of flow disruptions with patient, staff, and process outcomes: a prospective observational study of robotic-assisted radical prostatectomies. Surg Endosc 37, 6964–6974 (2023). https://doi.org/10.1007/s00464-023-10162-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10162-2