Abstract
Background
Human epidermal growth factor receptor 2 (HER2) expression in gastric cancer is highly heterogeneous. Therefore, it is important to take endoscopic samples from appropriate tumor sites.
Methods
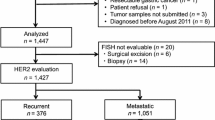
Between January 2008 and April 2015, patients with gastric or gastroesophageal junction cancer with histologically confirmed adenocarcinoma were included. Surgical samples or endoscopic biopsy samples were examined for HER2 using immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH). Tissues were considered to be HER2 positive when either assessment revealed either an IHC score of 3+ or an IHC score of 2+ accompanied by a positive FISH result. Endoscopic findings were retrieved in all cases where available, and we examined the portion from which a biopsy was obtained.
Results
Out of the 612 patients included in the study, 104 (17%) were HER2 positive. The proportion of HER2-positive gastric tumors with differentiated (vs. undifferentiated) histology was significantly higher (29 vs. 6%, respectively; p < 0.001). The HER2-positive rate of papillary adenocarcinomas (vs. tubular) was particularly high (62%, 8/13; p = 0.023). The proportion of HER2-positive gastric tumors of Borrmann classification 0 or 1 was significantly higher than that of tumors of classified as 2, 3, or 4 (45 vs. 16%, respectively; p < 0.001). The HER2-positive rates per biopsy specimen from the superficial spreading portion, ulcer mound, ulcer bed, and mass portion were 100, 91, 45, and 100%, respectively.
Conclusions
HER2-positive gastric cancer tends to be associated with a differentiated histology, particularly papillary adenocarcinoma, and a Borrmann classification of 0 or 1 tumors. Based on these endoscopic findings, it is important to recognize the superficial spreading portion and the mass portion of gastric malignancies.

Similar content being viewed by others
References
Global Burden of Disease Cancer C, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, Hay RJ, Hunter-Merrill R, Huynh C, Hosgood HD, Johnson CO, Jonas JB, Khubchandani J, Kumar GA, Kutz M, Lan Q, Larson HJ, Liang X, Lim SS, Lopez AD, MacIntyre MF, Marczak L, Marquez N, Mokdad AH, Pinho C, Pourmalek F, Salomon JA, Sanabria JR, Sandar L, Sartorius B, Schwartz SM, Shackelford KA, Shibuya K, Stanaway J, Steiner C, Sun J, Takahashi K, Vollset SE, Vos T, Wagner JA, Wang H, Westerman R, Zeeb H, Zoeckler L, Abd-Allah F, Ahmed MB, Alabed S, Alam NK, Aldhahri SF, Alem G, Alemayohu MA, Ali R, Al-Raddadi R, Amare A, Amoako Y, Artaman A, Asayesh H, Atnafu N, Awasthi A, Saleem HB, Barac A, Bedi N, Bensenor I, Berhane A, Bernabe E, Betsu B, Binagwaho A, Boneya D, Campos-Nonato I, Castaneda-Orjuela C, Catala-Lopez F, Chiang P, Chibueze C, Chitheer A, Choi JY, Cowie B, Damtew S, das Neves J, Dey S, Dharmaratne S, Dhillon P, Ding E, Driscoll T, Ekwueme D, Endries AY, Farvid M, Farzadfar F, Fernandes J, Fischer F, TT GH, Gebru A, Gopalani S, Hailu A, Horino M, Horita N, Husseini A, Huybrechts I, Inoue M, Islami F, Jakovljevic M, James S, Javanbakht M, Jee SH, Kasaeian A, Kedir MS, Khader YS, Khang YH, Kim D, Leigh J, Linn S, Lunevicius R, El Razek HMA, Malekzadeh R, Malta DC, Marcenes W, Markos D, Melaku YA, Meles KG, Mendoza W, Mengiste DT, Meretoja TJ, Miller TR, Mohammad KA, Mohammadi A, Mohammed S, Moradi-Lakeh M, Nagel G, Nand D, Le Nguyen Q, Nolte S, Ogbo FA, Oladimeji KE, Oren E, Pa M, Park EK, Pereira DM, Plass D, Qorbani M, Radfar A, Rafay A, Rahman M, Rana SM, Soreide K, Satpathy M, Sawhney M, Sepanlou SG, Shaikh MA, She J, Shiue I, Shore HR, Shrime MG, So S, Soneji S, Stathopoulou V, Stroumpoulis K, Sufiyan MB, Sykes BL, Tabares-Seisdedos R, Tadese F, Tedla BA, Tessema GA, Thakur JS, Tran BX, Ukwaja KN, Uzochukwu BSC, Vlassov VV, Weiderpass E, Wubshet Terefe M, Yebyo HG, Yimam HH, Yonemoto N, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zenebe ZM, Murray CJL, Naghavi M (2017) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol 3:524–548
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK, To GATI. (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697
Soularue E, Cohen R, Tournigand C, Zaanan A, Louvet C, Bachet JB, Hentic O, Samalin E, Chibaudel B, de Gramont A, Andre T, for G (2015) Efficacy and safety of trastuzumab in combination with oxaliplatin and fluorouracil-based chemotherapy for patients with HER2-positive metastatic gastric and gastro-oesophageal junction adenocarcinoma patients: a retrospective study. Bull Cancer 102:324–331
Gravalos C, Gomez-Martin C, Rivera F, Ales I, Queralt B, Marquez A, Jimenez U, Alonso V, Garcia-Carbonero R, Sastre J, Colomer R, Cortes-Funes H, Jimeno A (2011) Phase II study of trastuzumab and cisplatin as first-line therapy in patients with HER2-positive advanced gastric or gastroesophageal junction cancer. Clin Transl Oncol 13:179–184
Jorgensen JT, Hersom M (2012) HER2 as a prognostic marker in gastric cancer—a systematic analysis of data from the literature. J Cancer 3:137–144
Kim MA, Lee HJ, Yang HK, Bang YJ, Kim WH (2011) Heterogeneous amplification of ERBB2 in primary lesions is responsible for the discordant ERBB2 status of primary and metastatic lesions in gastric carcinoma. Histopathology 59:822–831
Grabsch H, Sivakumar S, Gray S, Gabbert HE, Muller W (2010) HER2 expression in gastric cancer: rare, heterogeneous and of no prognostic value—conclusions from 924 cases of two independent series. Cell Oncol 32:57–65
Grillo F, Fassan M, Ceccaroli C, Giacometti C, Curto M, Zagonel V, Ceppa P, Nitti D, Castoro C, Fiocca R, Rugge M, Mastracci L (2013) The reliability of endoscopic biopsies in assessing HER2 status in gastric and gastroesophageal junction cancer: a study comparing biopsies with surgical samples. Transl Oncol 6:10–16
Kim KC, Koh YW, Chang HM, Kim TH, Yook JH, Kim BS, Jang SJ, Park YS (2011) Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol 18:2833–2840
Hofmann M, Stoss O, Shi D, Buttner R, van de Vijver M, Kim W, Ochiai A, Ruschoff J, Henkel T (2008) Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 52:797–805
Lordick F, Shitara K, Janjigian YY (2017) New agents on the horizon in gastric cancer. Ann Oncol 28:1767–1775
Park JS, Rha SY, Chung HC, Jung M, Kim KH, Jun HJ, Kim H, An JY, Kim HI, Cheong JH, Hyung WJ, Noh SH, Kim HS (2015) Clinicopathological features and prognostic significance of HER2 expression in gastric cancer. Oncology 88:147–156
Laboissiere RS, Buzelin MA, Balabram D, De Brot M, Nunes CB, Rocha RM, Cabral MM, Gobbi H (2015) Association between HER2 status in gastric cancer and clinicopathological features: a retrospective study using whole-tissue sections. BMC Gastroenterol 15:157
He C, Bian XY, Ni XZ, Shen DP, Shen YY, Liu H, Shen ZY, Liu Q (2013) Correlation of human epidermal growth factor receptor 2 expression with clinicopathological characteristics and prognosis in gastric cancer. World J Gastroenterol 19:2171–2178
Siewert JR, Stein HJ (1998) Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 85:1457–1459
Kataoka Y, Okabe H, Yoshizawa A, Minamiguchi S, Yoshimura K, Haga H, Sakai Y (2013) HER2 expression and its clinicopathological features in resectable gastric cancer. Gastric Cancer 16:84–93
Gravalos C, Jimeno A (2008) HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol 19:1523–1529
Lordick F, Kang Y-K, Chung H-C, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, Park JO, Sawaki A, Celik I, Götte H, Melezínková H, Moehler M (2013) Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 14:490–499
Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K, Muro K, Kim YH, Ferry D, Tebbutt NC, Al-Batran SE, Smith H, Costantini C, Rizvi S, Lebwohl D, Van Cutsem E (2013) Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol 31:3935–3943
Aizawa M, Nagatsuma AK, Kitada K, Kuwata T, Fujii S, Kinoshita T, Ochiai A (2014) Evaluation of HER2-based biology in 1,006 cases of gastric cancer in a Japanese population. Gastric Cancer 17:34–42
Yano T, Doi T, Ohtsu A, Boku N, Hashizume K, Nakanishi M, Ochiai A (2006) Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep 15:65–71
Ruschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G (2012) HER2 testing in gastric cancer: a practical approach. Mod Pathol 25:637–650
Warneke VS, Behrens HM, Boger C, Becker T, Lordick F, Ebert MP, Rocken C (2013) Her2/neu testing in gastric cancer: evaluating the risk of sampling errors. Ann Oncol 24:725–733
Gullo I, Grillo F, Molinaro L, Fassan M, De Silvestri A, Tinelli C, Rugge M, Fiocca R, Mastracci L (2015) Minimum biopsy set for HER2 evaluation in gastric and gastro-esophageal junction cancer. Endosc Int Open 3::E165-170
Stout AP (1942) Superficial spreading type of carcinoma of the stomach. Arch Surg 44:630–636
Okabayashi T, Kobayashi M, Sugimoto T, Akimori T, Namikawa T, Okamoto K, Maeda H, Araki K (2006) Clinicopathological features of type 1 gastric carcinoma: the need to be cautious of superficial lesion surrounding type 1 gastric carcinoma. Hepatogastroenterology 53:313–316
Lee S, de Boer WB, Fermoyle S, Platten M, Kumarasinghe MP (2011) Human epidermal growth factor receptor 2 testing in gastric carcinoma: issues related to heterogeneity in biopsies and resections. Histopathology 59:832–840
Park SR, Park YS, Ryu MH, Ryoo BY, Woo CG, Jung HY, Lee JH, Lee GH, Kang YK (2016) Extra-gain of HER2-positive cases through HER2 reassessment in primary and metastatic sites in advanced gastric cancer with initially HER2-negative primary tumours: results of GASTric cancer HER2 reassessment study 1 (GASTHER1). Eur J Cancer 53:42–50
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Kohei Shitara reports personal fees from Astellas Pharma, grants and personal fees from Lilly, personal fees from Bristol-Myers Squibb, personal fees from Takeda, personal fees from Pfizer, grants and personal fees from Ono Pharmaceutical, personal fees from Novartis, personal fees from Abbvie, personal fees from Yakult, grants from Dainippon Sumitomo Pharma, grants from MSD, grants from Daiichi Sankyo, grants from Taiho Pharmaceutical, grants from Chugai Pharma, outside the submitted work. Takahiro Kinoshita reports personal fees from Olympus, personal fees from Johnson & Johnson, personal fees from Medtronic, personal fees from Yakult Pharma, personal fees from Taiho Pharma, personal fees from Eli Lilly, outside the submitted work. Yasuhiro Oono, Takeshi Kuwata, Kenji Takashima, Yusuke Yoda, Hiroaki Ikematsu, and Tomonori Yano have no conflict of interests or financial ties to disclose.
Ethical approval
The study protocol was approved by the medical ethics committee of the National Cancer Center Hospital East (2017-053). Patient informed consent was waived due to the retrospective design of the study. This study was performed in accordance with the ethical principles outlined in the Declaration of Helsinki.
Rights and permissions
About this article
Cite this article
Oono, Y., Kuwata, T., Takashima, K. et al. Clinicopathological features and endoscopic findings of HER2-positive gastric cancer. Surg Endosc 32, 3964–3971 (2018). https://doi.org/10.1007/s00464-018-6138-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6138-8