Abstract
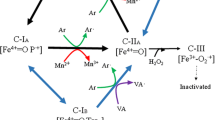
The cDNAs of six manganese-dependent peroxidases (MnPs) were isolated from white-rot fungus Polyporus brumalis. The MnP proteins shared similar properties with each other in terms of size (approximately 360–365 amino acids) and primary structure, showing 62–96 % amino acid sequence identity. RT-PCR analysis indicated that these six genes were predominantly expressed in shallow stationary culture (SSC) in a liquid medium. Gene expression was induced by treatment with dibutyl phthalate (DBP) and wood chips. Expression of pbmnp4 was strongly induced by both treatments, whereas that of pbmnp5 was induced only by DBP, while pbmnp6 was induced by wood chips only. Then, we overexpressed pbmnp4 in P. brumalis under the control of the GPD promoter. Overexpression of pbmnp4 effectively increased MnP activity; the transformant that had the highest MnP activity also demonstrated the most effective decolorization of Remazol Brilliant Blue R dye. Identification of MnP cDNAs can contribute to the efficient production of lignin-degradation enzymes and may lead to utilization of basidiomycetous fungi for degradation of lignin and numerous recalcitrant xenobiotics.






Similar content being viewed by others
References
Bumpus JA, Tien M, Wright D, Aust SD (1985) Oxidation of persistent environmental pollutants by a white rot fungus. Science 228(4706):1434–1436
Glenn JK, Gold MH (1985) Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys 242(2):329–341
Hammel KE, Kalyanaraman B, Kirk TK (1986) Substrate free radicals are intermediates in ligninase catalysis. Proc Natl Acad Sci USA 83(11):3708–3712
Wariishi H, Akileswaran L, Gold MH (1988) Manganese peroxidase from the basidiomycete Phanerochaete chrysosporium: spectral characterization of the oxidized states and the catalytic cycle. Biochemistry 12(14):5365–5370
Michel FC Jr, Dass SB, Grulke EA, Reddy CA (1991) Role of manganese peroxidases and lignin peroxidases of Phanerochaete chrysosporium in the decolorization of kraft bleach plant effluent. Appl Environ Microbiol 57(8):2368–2375
Paice MG, Reid ID, Bourbonnais R, Archibald FS, Jurasek L (1993) Manganese peroxidase, produced by Trametes versicolor during pulp bleaching, demethylates and delignifies Kraft pulp. Appl Environ Microbiol 59(2):260–265
Urzúa U, Fernando-Larrondo L, Lobos S, Larraín J, Vicuña R (1995) Oxidation reactions catalyzed by manganese peroxidase isoenzymes from Ceriporiopsis subvermispora. FEBS Lett 4(2):132–136
Sedighi M, Karimi A, Vahabzadeh F (2009) Involvement of ligninolytic enzymes of Phanerochaete chrysosporium in treating the textile effluent containing Astrazon Red FBL in a packed-bed bioreactor. J Hazard Mater 30(1–3):88–93
Tien M, Kirk TK (1984) Lignin-degrading enzyme from Phanerochaete chrysosporium: purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc Natl Acad Sci USA 81(8):2280–2284
Gold MH, Alic M (1993) Molecular biology of the lignin-degrading basidiomycete Phanerochaete chrysosporium. Microbiol Rev 57(3):605–622
Pasti-Grigsby MB, Paszczynski A, Goszczynski S, Crawford DL, Crawford RL (1992) Influence of aromatic substitution patterns on azo dye degradability by Streptomyces spp. and Phanerochaete chrysosporium. Appl Environ Microbiol 58(11):3605–3613
Gill PK, Arora DS, Chander M (2002) Biodecolourization of azo and triphenylmethane dyes by Dichomitus squalens and Phlebia spp. J Ind Microbiol Biotechnol 28(4):201–203
Lobos S, Larraín J, Salas L, Cullen D, Vicuña R (1994) Isoenzymes of manganese-dependent peroxidase and laccase produced by the lignin-degrading basidiomycete Ceriporiopsis subvermispora. Microbiology 140(10):2691–2698
Tien M, Tu C-PD (1987) Cloning and sequencing of a cDNA for a ligninase from Phanerochaete chrysosporium. Nature 326(6112):520–523
Pribnow D, Mayfield MB, Nipper VJ, Brown JA, Gold MH (1989) Characterization of a cDNA encoding a manganese peroxidase, from the lignin-degrading basidiomycete Phanerochaete chrysosporium. J Biol Chem 264(9):5036–5040
Dass SB, Reddy CA (1990) Characterization of extracellular peroxidases produced by acetate-buffered cultures of the lignin-degrading basidiomycete Phanerochaete chrysosporium. FEMS Microbiol Lett 57(3):221–224
Orth A, Rzhetskaya M, Cullen D, Tien M (1994) Characterization of a cDNA encoding a manganese peroxidase from Phanerochaete chrysosporium: genomic organization of lignin and manganese peroxidase genes. Gene 148(1):161–165
Kamitsuji H, Honda Y, Watanabe T, Kuwahara M (2004) Production and induction of manganese peroxidase isozymes in a white-rot fungus Pleurotus ostreatus. Appl Microbiol Biotechnol 65(3):287–294
Johansson T, Nymann P, Cullen D (2002) Differential regulation of mnp2, a new manganese peroxidase encoding gene from the ligninolytic fungus Trametes versicolor PRL572. Appl Environ Microbiol 68(4):2077–2080
Manubens A, Avila M, Canessa P, Vicuna R (2003) Differential regulation of genes encoding manganese peroxidase (MnP) in the basidiomycete Ceriporiopsis subvermispora. Curr Genet 43(6):433–438
Hakala T, Hilden K, Maijala P, Olsson C, Hadakka A (2006) Differential regulation of manganese peroxidases and characterization of two variable MnP encoding genes in the white rot fungus Physisporinus rivulosus. Appl Microbiol Biotechnol 73(4):839–849
Pease EA, Tien M (1992) Heterogeneity and regulation of manganese peroxidases from Phanerochaete chrysosporium. J Bacteriol 174(11):3532–3540
Kirk TK, Tien M, Faison BD (1984) Biochemistry of the oxidation of lignin by Phanerochaete chrysosporium. Biotechnol Adv 2(2):183–199
Martinez D, Larrondo LF, Putnam N, Sollewijm Gelpke MD, Huang K, Chapman J, Jelfenbein KG, Ramaiya P, Detter JC, Larimer F et al (2004) Genome sequence of the lignocellulose degrading fungus Phanerochaete chrysosporium strain RP78. Nat Biotechnol 22(6):695–697
Kim Y, Yeo S, Kum J, Song HG, Choi HT (2005) Cloning of a manganese peroxidase cDNA gene repressed by manganese in Trametes versicolor. J Microbiol 43(6):569–571
Yeo S, Park N, Song HG, Choi HT (2007) Generation of a transformant showing higher manganese peroxidase (MnP) activity by overexpression of MnP gene in Trametes versicolor. J Microbiol 45(3):213–218
Han M, Choi H, Song HG (2004) Degradation of phenanthrene by Trametes versicolor and its laccase. J Microbiol 42(2):94–98
Lee SM, Lee JW, Koo BW, Kim MK, Choi DH, Choi IG (2007) Dibutyl phthalate biodegradation by the white rot fungus, Polyporus brumalis. Biotechnol Bioeng 97(6):1516–1522
Lee SM, Park KR, Lee SS, Kim M, Choi IG (2005) Biodegradation of phthalic acid by white rot fungus, Polyporus brumalis. Mokchae Konghak 33(1):48–57
Lee JW, Gwak KS, Park JY, Park MJ, Choi DH, Kwon M, Choi IG (2007) Biological pretreatment of softwood Pinus densiflora by three white rot fungi. J Microbiol 45(6):485–491
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Leem Y, Kim S, Ross I, Choi H (1999) Transformation and laccase mutant isolation in Coprinus congregatus by restriction enzyme-mediated integration. FEMS Microbiol Lett 172:35–40
Carles R, Pere P (2006) Specific use of start codons and cellular localization of splice variants of human phosphodiesterase 9A gene. BMC Mol Biol 7(39):1–9
Nguyen PS (2009) The A312L 5′-UTR of Chlorella virus PBCV-1 is a translational enhancer in Arabidopsis thaliana. Virus Res 40(1–2):138–146
Canales M, Lobos S, Vicuña R (1998) Molecular modeling of manganese peroxidase from the lignin-degrading fungus Ceriporiopsis subvermispora and structural comparison with other peroxidases. Elec J Biotech 1(2):96–102
Sundaramoorthy M, Kishi K, Gold MH, Poulos TL (1997) Crystal structures of substrate binding site mutants of manganese peroxidase. J Biol Chem 272(28):17574–17580
Schoemaker HE, Lundell TK, Floris R, Glumoff T, Winterhalter KH, Piontek K (1994) Do carbohydrates play a role in lignin peroxidase cycle? Redox catalysis in the endergonic region of the driving force. Bioorg Med Chem 2(6):509–519
Choinowski T, Blodig W, Winterhalter K, Piontek K (1999) The crystal structure of lignin peroxidase at 1.70 Å resolution reveals a hydroxyl group on the Cb of tryptophan 171: a novel radical site formed during redox cycle. J Mol Biol 286(3):809–827
Pasti MB, Crawford DL (1991) Relationships between the abilities of Streptomycetes to decolorize three anthrone-type dyes and to degrade lignocelluloses. Can J Microbiol 37:902–907
Kum H, Lee S, Ryu S, Choi HT (2011) Degradation of endocrine disrupting chemicals by genetic transformants with two lignin degrading enzymes in Phlebia tremellosa. J Microbiol 49(5):824–827
Ruiz-Dueñas FJ, Morales M, Garcia E, Miki Y, Martinez MJ, Martinez AT (2009) Substrate oxidation sites in versatile peroxidase and other basidiomycete peroxidases. J Exp Bot 60(2):441–452
Acknowledgments
This study was carried out with the grant funded by Korea Forest Research Institute.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ryu, SH., Kim, B., Kim, M. et al. Molecular characterization of manganese peroxidases from white-rot fungus Polyporus brumalis . Bioprocess Biosyst Eng 37, 393–400 (2014). https://doi.org/10.1007/s00449-013-1004-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-013-1004-5