Abstract
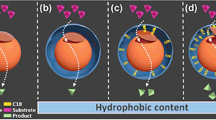
To enhance penicillin acylase (PA) performance, it was immobilized in mesocellular silica foams (MCFs) depended on macromolecular crowding and applied to catalysis in non-aqueous medium. Ficoll 70, dextran 10,000, dextran 40,000 and bovine serum albumin were co-assembled with PA. It was observed that specific activity of PA assembled in MCFs with dextran 10,000 in 80% cyclohexane (v/v) was 233.2 U/mg, 200% as that of PA assembled in MCFs in 80% cyclohexane and 323.5% as that of free PA in full aqueous medium. As content of alkane increased, activity of PA in MCFs with macromolecules varied slightly. In addition, PA co-immobilized with dextran 10 in MCFs retained 58.2% of its initial activity after heating at 50°C for 4 h, 1.2 times higher than that of PA immobilized alone in MCFs. The results showed that macromolecular crowding was favorable for immobilization of PA and its catalysis in suitable aqueous–organic medium.




Similar content being viewed by others
References
Chandel AK, Rao LV, Narasu ML et al (2008) The realm of penicillin G acylase in beta-lactam antibiotics. Enzyme Microb Technol 42:199–207
Parmar A, Kumar H, Marwaha S et al (2000) Advances in enzymatic transformation of penicillins to 6-aminopenicillanic acid (6-APA). Biotechnol Adv 18:289–301
Cao LQ, van Rantwijk F, Sheldon RA (2000) Cross-linked enzyme aggregates: a simple and effective method for the immobilization of penicillin acylase. Org Lett 2:1361–1364
Kallenberg AI, van Rantwijk F, Sheldon RA (2005) Immobilization of penicillin G acylase: the key to optimum performance. Adv Synth Catal 347:905–926
Wang AM, Wang H, Zhu SM et al (2008) An efficient immobilizing technique of penicillin acylase with combining mesocellular silica foams support and p-benzoquinone cross linker. Bioprocess Biosyst Eng 31:509–517
Wilson L, Illanes A, Abian O et al (2004) Co-aggregation of penicillin G acylase and polyionic polymers: an easy methodology to prepare enzyme biocatalysts stable in organic media. Biomacromolecules 5:852–857
Ferreira JS, Straathof AJJ, Franco TT et al (2004) Activity and stability of immobilized penicillin amidase at low pH values. J Mol Catal B Enzym 27:29–35
Cao XJ, Wu XY, Fonseca LJP et al (2004) Production of 6-aminopenicillanic acid in aqueous two-phase systems by recombinant Escherichia coli with intracellular penicillin acylase. Biotechnol Lett 26:97–101
Abian O, Mateo C, Fernandez-Lorente G et al (2003) Improving the industrial production of 6-APA: enzymatic hydrolysis of penicillin G in the presence of organic solvents. Biotechnol Prog 19:1639–1642
Montes T, Grazu V, Manso I et al (2007) Improved stabilization of genetically modified penicillin G acylase in the presence of organic cosolvents by co-immobilization of the enzyme with polyethyleneimine. Adv Synth Catal 349:459–464
Jiang M, Guo ZH (2007) Effects of macromolecular crowding on the intrinsic catalytic efficiency and structure of enterobactin-specific isochorismate synthase. J Am Chem Soc 129:730–731
Kuzu H, Altikatoglu M (2008) A study for HRP-dextran complexes with enhanced stabilities. FEBS J 275:410
Klibanov AM (2001) Improving enzymes by using them in organic solvents. Nature 409:241–246
Wang ZL, Guo YJ, Bao D et al (2006) Direct extraction of phenylacetic acid from immobilised enzymatic hydrolysis of penicillin G with cloud point extraction. J Chem Technol Biotechnol 81:560–565
Wyss A, Seitert H, von Stockar U et al (2005) Novel reactive perstraction system applied to the hydrolysis of penicillin G. Biotechnol Bioeng 91:227–236
Wang L, Wang ZL, Xu JH et al (2006) An eco-friendly and sustainable process for enzymatic hydrolysis of penicillin G in cloud point system. Bioprocess Biosyst Eng 29:157–162
Schmidt-Winkel P, Lukens WW, Zhao DY et al (1999) Mesocellular siliceous foams with uniformly sized cells and windows. J Am Chem Soc 121:254–255
Wang AM, Liu MQ, Wang H et al (2008) Improving enzyme immobilization in mesocellular siliceous foams by microwave irradiation. J Biosci Bioeng 106:286–291
Bradford MMA (1976) Rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–250
Shewale JG, Kumar KK, Ambekar GR (1987) Evaluation of determination of 6-aminopenicillanic acid by p-dimethylaminobenzaldehyde. Biotechnol Tech 1:69–72
Ellis RJ, Minton AP (2003) Cell biology—join the crowd. Nature 425:27–28
Minh DDL, Chang CE, Trylska J et al (2006) The influence of macromolecular crowding on HIV-1 protease internal dynamics. J Am Chem Soc 128:6006–6007
Ping GH, Yuan JM (2004) A study of macromolecular crowding effects on protein dynamics using lattice model. Biophys J 86:497A
Frauenfelder H (2008) What determines the speed limit on enzyme catalysis? Nat Chem Biol 4:21–22
Perutz MF (1978) Electrostatics effects in proteins. Science 201:1187–1191
Affleck R, Haynes CA, Clark DS (1992) Solvent dielectric effects on protein dynamics. Proc Natl Acad Sci USA 89:5167–5170
Affleck R, Xu ZF, Suzawa V et al (1992) Enzymatic catalysis and dynamics in low water environments. Proc Natl Acad Sci USA 89:1100–1104
Yan M, Ge J, Liu Z et al (2006) Encapsulation of single enzyme in nanogel with enhanced biocatalytic activity and stability. J Am Chem Soc 128:11008–11009
Arroyo M, Torres R, de la Mata I et al (1999) Interaction of penicillin V acylase with organic solvents: catalytic activity modulation on the hydrolysis of penicillin V. Enzyme Microb Technol 25:378–383
Wang QG, Gao QM, Shi JL (2004) Enhanced catalytic activity of hemoglobin in organic solvents by layered titanate immobilization. J Am Chem Soc 126:14346–14347
Bindhu LV, Abraham TE (2003) Preparation and kinetic studies of surfactant-horseradish peroxidase ion paired complex in organic media. Biochem Eng J 15:47–57
Wang XQ, Lu DN, Austin R et al (2007) Protein refolding assisted by periodic mesoporous organosilicas. Langmuir 23:5735–5739
Du F, Zhou Z, Mo ZY et al (2006) Mixed macromolecular crowding accelerates the refolding of rabbit muscle creatine kinase: implications for protein folding in physiological environments. J Mol Biol 364:469–482
Moran-Zorzano MT, Viale A, Munoz F et al (2007) Escherichia coli AspP activity is enhanced by macromolecular crowding and by both glucose-1,6-bisphosphate and nucleotide-sugars. FEBS Lett 581:1035–1040
Mateo C, Abian O, Fernandez-Lorente G et al (2002) Epoxy sepabeads: a novel epoxy support for stabilization of industrial enzymes via very intense multipoint covalent attachment. Biotechnol Prog 18:629–634
Lopez-Gallego F, Betancor L, Hidalgo A et al (2004) Optimization of an industrial biocatalyst of glutaryl acylase: stabilization of the enzyme by multipoint covalent attachment onto new amino-epoxy Sepabeads. J Biotechnol 111:219–227
Lopez-Gallego F, Montes T, Fuentes M et al (2005) Improved stabilization of chemically aminated enzymes via multipoint covalent attachment on glyoxyl supports. J Biotechnol 116:1–10
Hari Krishna S (2002) Developments and trends in enzyme catalysis in nonconventional media. Biotechnol Adv 20:239–267
Minton AP (2006) How can biochemical reactions within cells differ from those in test tubes? J Cell Sci 119:2863–2869
Sasahara K, McPhie P, Minton AP (2003) Effect of dextran on protein stability and conformation attributed to macromolecular crowding. J Mol Biol 326:1227–1237
Garg S, Kumar A (2007) Immobilization of starch phosphorylase from seeds of Indian millet (Pennisetum typhoides) variety KB 560. Afr J Biotechnol 6:2715–2720
Acknowledgments
This work was financially supported by the National High Technology Research and Development Program of China (863 Program, No. 2006AA02Z211), National Natural Science Foundation of China (20376034), Natural Science Foundation of Jiangsu Province of China (BK2006181) and Foundation of Jiangsu Province of China for College Postgraduate Students in Innovation Engineering (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xue, J., Wang, A., Zhou, C. et al. Penicillin acylase immobilization depending on macromolecular crowding and catalysis in aqueous–organic medium. Bioprocess Biosyst Eng 32, 765–772 (2009). https://doi.org/10.1007/s00449-009-0301-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-009-0301-5