Abstract
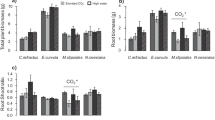
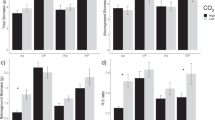
Eriophorum vaginatum is a characteristic species of northern peatlands and a keystone plant for cutover bog restoration. Understanding the factors affecting E. vaginatum seedling establishment (i.e. growth dynamics and allocation) under global change has practical implications for the management of abandoned mined bogs and restoration of their C-sequestration function. We studied the responses of leaf dynamics, above- and belowground biomass production of establishing seedlings to elevated CO2 and N. We hypothesised that nutrient factors such as limitation shifts or dilutions would modulate growth stimulation. Elevated CO2 did not affect biomass, but increased the number of young leaves in spring (+400 %), and the plant vitality (i.e. number of green leaves/total number of leaves) (+3 %), both of which were negatively correlated to [K+] in surface porewater, suggesting a K-limited production of young leaves. Nutrient ratios in green leaves indicated either N and K co-limitation or K limitation. N addition enhanced the number of tillers (+38 %), green leaves (+18 %), aboveground and belowground biomass (+99, +61 %), leaf mass-to-length ratio (+28 %), and reduced the leaf turnover (−32 %). N addition enhanced N availability and decreased [K+] in spring surface porewater. Increased tiller and leaf production in July were associated with a doubling in [K+] in surface porewater suggesting that under enhanced N production is K driven. Both experiments illustrate the importance of tradeoffs in E. vaginatum growth between: (1) producing tillers and generating new leaves, (2) maintaining adult leaves and initiating new ones, and (3) investing in basal parts (corms) for storage or in root growth for greater K uptake. The K concentration in surface porewater is thus the single most important factor controlling the growth of E. vaginatum seedlings in the regeneration of selected cutover bogs.




Similar content being viewed by others
References
Aerts R, Wallén B, Malmer N (1992) Growth-limiting nutrients in Sphagnum-dominated bogs subject to low and high atmospheric nitrogen supply. J Ecol 80:131–140
Backéus I (1985) Aboveground production and growth dynamics of vascular bog plants in Central Sweden. Acta Phytogeogr Suec 74:1–98
Bassirirad H, Tissue DT, Reynolds JF, Chapin FS III (1996) Response of Eriophorum vaginatum to CO2 enrichment at different temperatures: effects on growth, root respiration and PO4 3− uptake kinetics. New Phytol 133:423–430
Berendse F, Rydin H, van Breemen N, Buttler A, Heijmans M, Hoosbeek MR, Lee J, Mitchell EAD, Saarnio S, Vasander H, Wallen B (2001) C sequestration is influenced by competitive shifts among plant species in Sphagnum bogs at elevated atmospheric CO2 and N deposition. Glob Change Biol 7:591–598
Bliss LC (1956) A comparison of plant development in microenvironments of arctic and alpine plants. Ecol Monogr 26:303–337
Buurman P, van Lagen B, Velthorst EJ (1996) Manual for soil and water analysis. Backhuys, Leiden
Chapin FS III, Shaver GR (1996) Physiological and growth responses of arctic plants to a field experiment simulating climatic change. Ecology 77:822–840
Cholewa E, Griffith M (2004) The unusual vascular structure of the corm of Eriophorum vaginatum: implications for efficient retranslocation of nutrients. J Exp Bot 55:731–741
Conroy JP (1992) Influence of elevated atmospheric CO2 concentrations on plant nutrition. Aust J Bot 40:445–456
Damman AWH (1978) Distribution and movement of elements in ombrotrophic peat bogs. Oikos 30:481–495
Farrar JF (1985) The respiratory source of CO2. Plant Cell Environ 8:427–438
Fitter AH, Graves JD, Wolfenden J, Self GK, Brown TK, Bogie D, Mansfield TA (1997) Root production and turnover and carbon budgets of two contrasting grasslands under ambient and elevated atmospheric carbon dioxide concentrations. New Phytol 137:247–255
Francez AJ (1995) Carbon and nitrogen dynamics in Carex-rostrata, Eriophorum-vaginatum and Calluna-vulgaris in Sphagnum peat of the Forez mountains (France). Can J Bot 73:121–129
Gebauer RLE, Tenhunen JD, Reynolds JF (1995) Soil aeration in relation to soil physical properties, nitrogen availability, and root characteristics within an arctic watershed. Plant Soil 178:37–48
Goodman GT, Perkins DF (1968a) The role of mineral nutrients in Eriophorum communities. III. Growth response to added inorganic elements in two E. vaginatum communities. J Ecol 56:667–683
Goodman GT, Perkins DF (1968b) The role of mineral nutrients in Eriophorum communities. IV. Potassium supply as a limiting factor in an E. vaginatum community. J Ecol 56:685–696
Gough L (2006) Neighbour effects on germination, survival, and growth in two arctic tundra plant communities. Ecography 29:44–56
Grosvernier P, Matthey Y, Buttler A (1995) Microclimate and physical properties of peat: new clues to the understanding of bog restoration process. In: Wheeler B, Shaw S, Foyt W, Robertson A (eds) The restoration of temperate wetlands. Wiley, Chichester, pp 437–445
Hoosbeek M, van Bremen N, Vasander H, Buttler A, Berendse F (2002) Potassium limits potential growth of bog vegetation under elevated atmospheric CO2 and N deposition. Glob Change Biol 8:1130–1138
Hunt R, Hand DW, Hannah MA, Neal AM (1991) Response to CO2 enrichment in 27 herbaceous species. Funct Ecol 5:410–421
Johnson DW, Ball JT, Walker RF (1997) Effects of CO2 and nitrogen fertilization on vegetation and soil nutrient content in juvenile ponderosa pine. Plant Soil 190:29–40
Jonasson S, Chapin FS III (1985) Significance of sequential leaf development for nutrient balance of the cotton sedge, Eriophorum vaginatum L. Oecologia 67:511–518
Kivimäki SK (2008) Carbon sink function of sedge and Sphagnum patches in a restored cut-away peatland: increased functional diversity leads to higher production. J Appl Ecol 45:921–929
Körner C (1995) Biodiversity and CO2: global change is under way. Gaia 4:234–243
Lambers H, Atkin O (1995) Regulation of carbon metabolism in roots. In: Madore MA, Lucas WJ (eds) Carbon partitioning and source-sink interactions in plants. The American Society of Plant Physiologists, Rockville, pp 226–238
Miglietta F, Hoosbeek MR, Foot J, Gigon F, Heijmans M, Peressotti A, Saarinen T, van Breemen N, Wallén B (2001) Spatial and temporal performance of the MiniFace (free Air CO2 enrichment) system on bog ecosystems in northern and central Europe. Environ Monit Assess 66:107–127
Mitchell AED, Buttler A, Grosvernier P, Rydin H, Siegenthaler A, Gobat JM (2002) Contrasted effects of increased N and CO2 levels on two keystone species in peatlands restoration and implications for global change. J Ecol 90:529–533
Mitchell EAD, Gilbert D, Buttler A, Amblard C, Grosvernier P, Gobat JM (2003) Structure of microbial communities in Sphagnum peatlands and effect of atmospheric carbon dioxide enrichment. Microb Ecol 46:187–199
NABEL (1995) Air Pollution 1994. Measurements made by the national survey network of atmospheric pollutants. Federal Service for Environment, Forest and Landscape, Bern
NABEL (2009) Air Pollution 2008. Measurements made by the national survey network of atmospheric pollutants. Federal Service for Environment, Forest and Landscape, Bern
Noble JC, Miller PC (1979) The population biology of plants with clonal growth. The morphology and structural demography of Carex arenaria. J Ecol 62:151–177
Oechel WC, Vourlitis GL (1996) Direct effects of elevated CO2 on arctic plant and ecosystem function. In: Koch GW, Mooney HA (eds) Carbon dioxide and terrestrial ecosystems. Academic Press, San Diego, pp 163–176
R Development Core Team (2008) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0. URL http://www.R-project.org
Rogers HH, Runion GB (1994) Plant responses to atmospheric CO2 enrichment with emphasis on roots and the rhizosphere. Environ Pollut 83:155–189
Schimel JP, Chapin FS III (1996) Tundra plant uptake of amino acid and NH4 + nitrogen in situ: plants compete well for amino acid N. Ecology 77:2142–2147
Shaver GR, Chapin FS III (1980) Response to fertilization by various plant growth forms in an Alaskan tundra: nutrient accumulation and growth. Ecology 61:662–675
Shaver GR, Chapin FS III, Gartner BL (1986) Factors limiting seasonal growth and peak biomass accumulation in Eriophorum vaginatum in Alaskan tussock tundra. J Bryology 74:257–278
Stulen I, Den Hertog J (1993) Root growth and functioning under atmospheric CO2 enrichment. Vegetatio 104(105):99–115
Sullivan PF, Sommerkorn M, Rueth HM, Nadelhoffer KJ, Saver GR, Welker JM (2007) Climate and species affect fine root production with long-term fertilization in acidic tussock tundra near Toolik Lake, Alaska. Oecologia 153:643–652
Taiz L, Zeiger E (1998) Plant physiology. Sinauer, Sunderland
Tamm CO (1954) Some observations on the nutrient turn-over in a bog community dominated by Eriophorum vaginatum L. Oikos 5:189–194
Taub DR, Wang XZ (2008) Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J Integr Plant Biol 50:1365–1374
Tissue DT, Oechel WC (1987) Response of Eriophorum vaginatum to elevated CO2 and temperature in the Alaskan tussock tundra. Ecology 68:401–410
van Heerwaarden LM, Toet S, Aerts R (2003) Nitrogen and phosphorus resorption efficiency and proficiency in six sub-arctic bog species after 4 years of nitrogen fertilization. J Ecol 91:1060–1070
van Wijk MT, Clemmensen KE, Shaver GR, Williams M, Callaghan TV, Chapin FS, Cornelissen JHC, Gough L, Hobbie SE, Jonasson S, Lee JA, Michelsen A, Press MC, Richardson SJ, Rueth H (2004) Long-term ecosystem level experiments at Toolik Lake, Alaska, and at Abisko, Northern Sweden: generalizations and differences in ecosystem and plant type responses to global change. Glob Change Biol 10:105–123
Vasander H (1982) Plant biomass and production in virgin, drained and fertilized sites in a raised bog in southern Finland. Ann Bot Fenn 19:103–125
Walinga I, van der Lee JJ, Houba VJG, Van Vark W, Novozamsky I (1995) Plant analysis manual. Kluwer, Dordrecht
Wein RW (1973) Biological flora of the British Isles. J Ecol 61:601–613
Wulff RD, Strain BR (1982) Effects of CO2 enrichment on growth and photosynthesis in Desmodium paniculatum. Can J Bot 60:1084–1109
Acknowledgments
This work was carried out in the framework of the EU-BERI (Bog Ecosystem Research Initiative). We thank Jacqueline Moret (University of Neuchâtel) and Prof. Jun Yu (SLU, Umeå, Sweden) for statistical advice, and Fabrizio Manco, Sylvie Marcacci, Oriane Grosvernier for technical assistance.
Conflict of interest
The authors declare that they have no conflict of interest and that experiments comply with the current laws of Switzerland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Zoe Cardon.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Siegenthaler, A., Buttler, A., Grosvernier, P. et al. Factors modulating cottongrass seedling growth stimulation to enhanced nitrogen and carbon dioxide: compensatory tradeoffs in leaf dynamics and allocation to meet potassium-limited growth. Oecologia 171, 557–570 (2013). https://doi.org/10.1007/s00442-012-2415-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2415-8