Abstract
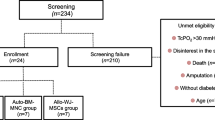
Peripheral artery disease (PAD) affects more than 230 million people worldwide, with approximately 11% of patients presenting with advanced-stage PAD or critical limb ischemia (CLI). To avoid or delay amputation, particularly in no-option CLI patients with infeasible or ineffective revascularization, new treatment strategies such as regenerative therapies should be developed. Mesenchymal stem cells (MSCs) are the most popular cell source in regenerative therapies. They possess significant characteristics such as angiogenic, anti-inflammatory, and immunomodulatory activities, which encourage their application in different diseases. This phase I clinical trial reports the safety, feasibility, and probable efficacy of the intramuscular administration of allogeneic Wharton’s jelly-derived MSCs (WJ-MSCs) in type 2 diabetes patients with CLI. Out of six screened patients with CLI, five patients were administered WJ-MSCs into the gastrocnemius, soleus, and the proximal part of the tibialis anterior muscles of the ischemic lower limb. The safety of WJ-MSCs injection was considered a primary outcome. Secondary endpoints included wound healing, the presence of pulse at the disease site, the absence of amputation, and improvement in visual analogue scale (VAS), pain-free walking time, and foot and ankle disability index (FADI). No patient experienced adverse events and foot or even toe amputation during the 6-month follow-up. Six months after the intervention, there were a significantly lower VAS score and significantly higher pain-free walking time and FADI score than the baseline, but no statistically significant difference was seen between other time points. In conclusion, allogeneic WJ-MSC transplantation in patients with CLI seems to be safe and effective.




Similar content being viewed by others
Availability of data and material
All data are available by corresponding author.
Abbreviations
- AE:
-
Adverse event
- CLI:
-
Critical limb ischemia
- FADI:
-
Foot and ankle disability index
- NO-CLI:
-
No-option CLI
- VAS:
-
Visual analogue scale
- WJ-MSC:
-
Wharton’s jelly-derived mesenchymal stem cell
References
Acosta L, Hmadcha A, Escacena N, Pérez-Camacho I, de la Cuesta A, Ruiz-Salmeron R, Gauthier BR, Soria B (2013) Adipose mesenchymal stromal cells isolated from type 2 diabetic patients display reduced fibrinolytic activity. Diabetes 62:4266–4269
American Diabetes Association (2003) Peripheral arterial disease in people with diabetes. Diabetes Care 26:3333
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–966
Attanasio S, Snell J (2009) Therapeutic angiogenesis in the management of critical limb ischemia: current concepts and review. Cardiol Rev 17:115–120
Black JH III, LaMuraglia GM, Kwolek CJ, Brewster DC, Watkins MT, Cambria RP (2005) Contemporary results of angioplasty-based infrainguinal percutaneous interventions. J Vasc Surg 42:932–939
Cassidy FC, Shortiss C, Murphy CG, Kearns SR, Curtin W, De Buitléir C, O’Brien T, Coleman CM (2020) Impact of type 2 diabetes mellitus on human bone marrow stromal cell number and phenotypic characteristics. Int J Mol Sci 21:2476
Coats P, Wadsworth R (2005) Marriage of resistance and conduit arteries breeds critical limb ischemia. American Journal of Physiology-Heart and Circulatory Physiology 288:H1044–H1050
Faglia E, Favales F, Quarantiello A, Calia P, Clelia P, Brambilla G, Rampoldi A, Morabito A (1998) Angiographic evaluation of peripheral arterial occlusive disease and its role as a prognostic determinant for major amputation in diabetic subjects with foot ulcers. Diabetes Care 21:625–630
Fang G, Jiang X, Fang Y, Pan T, Liu H, Ren B, Wei Z, Gu S, Chen B, Jiang J (2020) Autologous peripheral blood-derived stem cells transplantation for treatment of no-option angiitis-induced critical limb ischemia: 10-year management experience. Stem Cell Res Ther 11:1–12
Farzamfar S, Salehi M, Ehterami A, Naseri-Nosar M, Vaez A, Zarnani AH, Sahrapeyma H, Shokri M-R, Aleahmad M (2018) Promotion of excisional wound repair by a menstrual blood-derived stem cell-seeded decellularized human amniotic membrane. Biomed Eng Lett 8:393–398
Ferrer-Lorente R, Bejar MT, Tous M, Vilahur G, Badimon L (2014) Systems biology approach to identify alterations in the stem cell reservoir of subcutaneous adipose tissue in a rat model of diabetes: effects on differentiation potential and function. Diabetologia 57:246–256
Gu Y-q, Zhang J, Guo L-r, Qi L-x, Zhang S-w, Xu J, Li J-x, Luo T, Ji B-x, Li X-f (2008) Transplantation of autologous bone marrow mononuclear cells for patients with lower limb ischemia. Chin Med J 121:963–967
Gupta PK, Chullikana A, Parakh R, Desai S, Das A, Gottipamula S, Krishnamurthy S, Anthony N, Pherwani A, Majumdar AS (2013) A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med 11:1–11
Gupta PK, Krishna M, Chullikana A, Desai S, Murugesan R, Dutta S, Sarkar U, Raju R, Dhar A, Parakh R (2017) Administration of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells in critical limb ischemia due to Buerger’s disease: phase II study report suggests clinical efficacy. Stem Cells Transl Med 6:689–699
Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB (2006) ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American association for vascular surgery/society for vascular surgery, society for cardiovascular angiography and interventions, society for vascular medicine and biology, society of interventional radiology, and the ACC/AHA task force on practice guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease) endorsed by the American association of cardiovascular and pulmonary rehabilitation; national heart, lung, and blood institute; society for vascular nursing; TransAtlantic inter-society consensus; and vascular disease foundation. J Am Coll Cardiol 47:1239–1312
Hoang DM, Pham PT, Bach TQ, Ngo AT, Nguyen QT, Phan TT, Nguyen GH, Le PT, Hoang VT, Forsyth NR (2022) Stem cell-based therapy for human diseases. Signal Transduct Target Ther 7:272
Huang Q, Shu H, Zeng C, Qiu P, Xiong X, Lu X (2022) Endovascular revascularisation versus surgical revascularisation in patients with lower limb atherosclerosis obliterans: a protocol for systematic review and meta-analysis with trial sequential analysis and meta-regression. BMJ Open 12:e066903
Keymel S, Heinen Y, Balzer J, Rassaf T, Kelm M, Lauer T, Heiss C (2011) Characterization of macro-and microvascular function and structure in patients with type 2 diabetes mellitus. Am J Cardiovasc Dis 1:68
Kinnaird T, Stabile E, Burnett M, Lee C, Barr S, Fuchs S, Epstein S (2004) Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res 94:678–685
Kreitner K-F, Kalden P, Neufang A, Düber C, Krummenauer F, Küstner E, Laub G, Thelen M (2000) Diabetes and peripheral arterial occlusive disease: prospective comparison of contrast-enhanced three-dimensional MR angiography with conventional digital subtraction. Am J Roentgenol 174:171–179
Kwiatkowski T,Zbierska-Rubinkiewicz K,Krzywoń J,Szkółka Ł,Kuczmik W,Majka M,Maga P,Drelicharz Ł,Musiałek P, Trystuła M (2022) Cellular therapies in no-option critical limb ischemia: present status and future directions. Advances in Interventional Cardiology/Postępy w Kardiologii Interwencyjnej. 18
Lawall H, Bramlage P, Amann B (2011) Treatment of peripheral arterial disease using stem and progenitor cell therapy. J Vasc Surg 53:445–453
Majmundar M, Patel KN, Doshi R, Anantha-Narayanan M, Kumar A, Reed GW, Puri R, Kapadia SR, Jaradat ZA, Bhatt DL (2022) Comparison of 6-month outcomes of endovascular vs surgical revascularization for patients with critical limb ischemia. JAMA Netw Open 5:e2227746–e2227746
Mamidi MK, Pal R, Dey S, Abdullah BJJB, Zakaria Z, Rao MS, Das AK (2012) Cell therapy in critical limb ischemia: current developments and future progress. Cytotherapy 14:902–916
Martin A, Komada MR, Sane DC (2003) Abnormal angiogenesis in diabetes mellitus. Med Res Rev 23:117–145
Matoba S, Tatsumi T, Murohara T, Imaizumi T, Katsuda Y, Ito M, Saito Y, Uemura S, Suzuki H, Fukumoto S (2008) Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J 156:1010–1018
Minteer DM, Young MT, Lin Y-C, Over PJ, Rubin JP, Gerlach JC, Marra KG (2015) Analysis of type II diabetes mellitus adipose-derived stem cells for tissue engineering applications. J Tissue Eng 6:2041731415579215
Muylaert DE, de Jong OG, Slaats GG, Nieuweboer FE, Fledderus JO, Goumans M-J, Hierck BP, Verhaar MC (2016) Environmental influences on endothelial to mesenchymal transition in developing implanted cardiovascular tissue-engineered grafts. Tissue Eng Part B Rev 22:58–67
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR, Group TIW (2007) Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 45:S5–S67
Powell RJ (2012) Update on clinical trials evaluating the effect of biologic therapy in patients with critical limb ischemia. J Vasc Surg 56:264–266
Pries A (2003) Reglin B, and Secomb TW. Structural response of microcirculatory networks to changes in demand: information transfer by shear stress. Am J Physiol Heart Circ Physiol 284:H2204–H2212
Qadura M, Terenzi DC, Verma S, Al-Omran M, Hess DA (2018) Concise review: cell therapy for critical limb ischemia: an integrated review of preclinical and clinical studies. Stem Cells 36:161–171
Qin H, Zhu X, Zhang B, Zhou L, Wang W (2016) Clinical evaluation of human umbilical cord mesenchymal stem cell transplantation after angioplasty for diabetic foot. Exp Clin Endocrinol Diabetes 124:497–503
Rennert RC, Sorkin M, Januszyk M, Duscher D, Kosaraju R, Chung MT, Lennon J, Radiya-Dixit A, Raghvendra S, Maan ZN (2014) Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res Ther 5:1–12
Ruiz-Salmeron R, De La Cuesta-Diaz A, Constantino-Bermejo M, Pérez-Camacho I, Marcos-Sánchez F, Hmadcha A, Soria B (2011) Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transplant 20:1629–1639
Saremi J, Zarei-Behjani Z, Vojoudi E, Ebrahimi-Barough S (2022) Evaluation of viability and cell attachment of human endometrial stem cells on electrospun silk scaffolds prepared under different degumming conditions and solvents. Regen Eng Transl Med 8:593–606
Shang Y, Guan H, Zhou F (2021) Biological characteristics of umbilical cord mesenchymal stem cells and its therapeutic potential for hematological disorders. Front Cell Dev Biol 9:570179
Sharma S, Pandey NN, Sinha M, Kumar S, Jagia P, Gulati GS, Gond K, Mohanty S, Bhargava B (2021) Randomized, double-blind, placebo-controlled trial to evaluate safety and therapeutic efficacy of angiogenesis induced by intraarterial autologous bone marrow–derived stem cells in patients with severe peripheral arterial disease. J Vasc Interv Radiol 32:157–163
Shirbaghaee Z, Heidari Keshel S, Rasouli M, Valizadeh M, Hashemi Nazari SS, Hassani M, Soleimani M (2023) Report of a phase 1 clinical trial for safety assessment of human placental mesenchymal stem cells therapy in patients with critical limb ischemia (CLI). Stem Cell Res Ther 14:1–16
Soria-Juan B, Escacena N, Capilla-Gonzalez V, Aguilera Y, Llanos L, Tejedo JR, Bedoya FJ, Juan V, De la Cuesta A, Ruiz-Salmerón R (2019) Cost-effective, safe, and personalized cell therapy for critical limb ischemia in type 2 diabetes mellitus. Front Immunol 10:1151
Wang SK, Green LA, Drucker NA, Motaganahalli RL, Fajardo A, Murphy MP (2018) Rationale and design of the Clinical and Histologic Analysis of Mesenchymal Stromal Cells in Am Putations (CHAMP) trial investigating the therapeutic mechanism of mesenchymal stromal cells in the treatment of critical limb ischemia. J Vasc Surg 68(176–181):e171
Wijnand JG, Teraa M, Gremmels H, van Rhijn-Brouwer FC, de Borst GJ, Verhaar MC, Group SS (2018) Rationale and design of the SAIL trial for intramuscular injection of allogeneic mesenchymal stromal cells in no-option critical limb ischemia. J Vasc Surg 67:656–661
Xiao X, Wu Z-C, Chou K-C (2011) A multi-label classifier for predicting the subcellular localization of gram-negative bacterial proteins with both single and multiple sites. Plos One 6:20592
Acknowledgements
The authors kindly thank the Regenerative Medicine, Organ Procurement and Transplantation Multi-Disciplinary Center based on Razi Hospital, Rasht for helping and supporting this study.
Funding
This study was supported by a grant from Guilan University of Medical Sciences (Trial registration: IRCT, 20211021052828N2. Registered May 15, 2022).
Author information
Authors and Affiliations
Contributions
MTA: conceptualization, investigation of the study, data analysis, writing the initial draft, verification of the final version of manuscript. HH: conceptualization, investigation of the study, verification of the final version of manuscript. HRA: conceptualization, investigation of the study, verification of the final version of manuscript. ZZB: conceptualization, verification of the final version of manuscript. SK: conceptualization, verification of the final version of manuscript. HB: conceptualization, verification of the final version of manuscript. SM: data analysis, verification of the final version of manuscript. SE: investigation of the study, writing the initial draft, verification of the final version of manuscript. MF: helping to gather data, verification of the final version of manuscript. EV: conceptualization, investigation of the study, data analysis, writing the initial draft, verification of the final version of manuscript, submitting the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study complied with the ethical standards of the Guilan University of Medical Sciences with this number: IR.GUMS.REC.1401.015 (IRCT NO. IRCT20211021052828N2). Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ashoobi, M.T., Hemmati, H., Aghayan, H.R. et al. Wharton’s jelly mesenchymal stem cells transplantation for critical limb ischemia in patients with type 2 diabetes mellitus: a preliminary report of phase I clinical trial. Cell Tissue Res 395, 211–220 (2024). https://doi.org/10.1007/s00441-023-03854-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-023-03854-7