Abstract
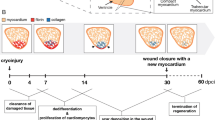
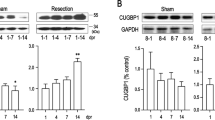
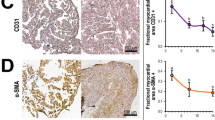
The adult mammalian heart is non-regenerative because cardiomyocytes withdraw from the cell cycle shortly after birth. Embryonic mammalian hearts, in which cardiomyocytes are genetically ablated in a salt-and-pepper–like pattern, regenerate due to compensation by residual cardiomyocytes. To date, it remains unknown whether or how transmural ventricular defects at the looped heart stage regenerate after cryoinjury. We established a cryoablation model in stage 16 chick embryonic hearts. In hearts at 5 h post cryoinjury (hpc), cryoinjury-induced defects were approximately 200 µm in width in the primitive ventricle; thereafter, the defect was filled with mesenchymal cells accumulating between the epicardium and endocardium. The defect began to regress at 4 days post cryoinjury (dpc) and disappeared around 9 dpc. Immunohistochemistry showed that there were no isl1-positive cells in either the scar tissue or residual cardiomyocytes. BrdU incorporation into residual cardiomyocytes was transiently downregulated in association with upregulation of p27 (Kip1), suggesting that cell cycle arrest occurred at G1-to-S transition immediately after cryoinjury. Estimated cell cycle length was examined, and the results showed that the shortest cell cycle length was 18 h at stages 19–23; it increased with development due to elongation of the G2-M-G1 phase and 30 h at stages 27–29. The S phase length was constant at 6–8 h. The cell cycle length was elongated immediately after cryoinjury, and it reversed at 1–2 dpc. Cryoablated transmural defects in the early embryonic heart were restored by compensation by residual myocytes.






Similar content being viewed by others
References
Alexiades MR, Cepko C (1996) Quantitative analysis of proliferation and cell cycle length during development of the rat retina. Dev Dyn 205:293–307. https://doi.org/10.1002/(sici)1097-0177(199603)205:3293::aid-aja93.0.co;2-d
Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J (2009) Evidence for cardiomyocyte renewal in humans. Science 324:98–102. https://doi.org/10.1126/science.1164680
Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM (2008) A myocardial lineage derives from Tbx18 epicardial cells. Nature 454:104-108 https://doi.org/10.1038/nature06969
Darehzereshki A, Rubin N, Gamba L, Kim J, Fraser J, Huang Y, Billings J, Mohammadzadeh R, Wood J, Warburton D, Kaartinen V, Lien CL (2015) Differential regenerative capacity of neonatal mouse hearts after cryoinjury. Dev Biol 399:91–99. https://doi.org/10.1016/j.ydbio.2014.12.018
de Boer BA, van den Berg G, de Boer PA, Moorman AF, Ruijter JM (2012a) Growth of the developing mouse heart: an interactive qualitative and quantitative 3D atlas. Dev Biol 368:203–213. https://doi.org/10.1016/j.ydbio.2012.05.001
de Boer BA, van den Berg G, Soufan AT, de Boer PA, Hagoort J, van den Hoff MJ, Moorman AF, Ruijter JM (2012b) Measurement and 3D-visualization of cell-cycle length using double labelling with two thymidine analogues applied in early heart development. PLoS One 7:e47719. https://doi.org/10.1371/journal.pone.0047719
Dobaczewski M, Chen W, Frangogiannis NG (2011) Transforming growth factor (TGF)-β signaling in cardiac remodeling. J Mol Cell Cardiol 51:600–606. https://doi.org/10.1016/j.yjmcc.2010.10.033
Drenckhahn JD, Schwarz QP, Gray S, Laskowski A, Kiriazis H, Ming Z, Harvey RP, Du XJ, Thorburn DR, Cox TC (2008) Compensatory growth of healthy cardiac cells in the presence of diseased cells restores tissue homeostasis during heart development. Dev Cell 15:521–533. https://doi.org/10.1016/j.devcel.2008.09.005
González-Rosa JM, Martín V, Peralta M, Torres M, Mercader N (2011) Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 138:1663–1674. https://doi.org/10.1242/dev.060897
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49-92
Hashimoto H, Yuasa S, Tabata H, Tohyama S, Hayashiji N, Hattori F, Muraoka N, Egashira T, Okata S, Yae K, Seki T, Nishiyama T, Nakajima K, Sakaue-Sawano A, Miyawaki A, Fukuda K (2014) Time-lapse imaging of cell cycle dynamics during development in living cardiomyocyte. J Mol Cell Cardiol 72:241–249. https://doi.org/10.1016/j.yjmcc.2014.03.020
Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD (1997) Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106:348–360. https://doi.org/10.1007/s004120050256
Jesty SA, Steffey MA, Lee FK, Breitbach M, Hesse M, Reining S, Lee JC, Doran RM, Nikitin AY, Fleischmann BK, Kotlikoff MI (2012) c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc Natl Acad Sci USA 109:13380–13385. https://doi.org/10.1073/pnas.1208114109
Jeter JR Jr, Cameron IL (1971) Cell proliferation patterns during cytodifferentiation in embryonic chick tissues: liver, heart and erythrocytes. J Embryol Exp Morphol 25:405–422
Jopling C, Sleep E, Raya M, Martí M, Raya A, Izpisúa Belmonte JC (2010) Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 464:606–609. https://doi.org/10.1038/nature08899
Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD (2010) Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature 464:601–605. https://doi.org/10.1038/nature08804
Lam NT, Sadek HA (2018) Neonatal Heart Regeneration: comprehensive literature review. Circulation 138:412–423. https://doi.org/10.1161/circulationaha.118.033648
Manner J (1999) Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat Rec 255:212–226. https://doi.org/10.1002/(sici)1097-0185(19990601)255:2212::aid-ar113.3.co;2-o
Matrone G, Taylor JM, Wilson KS, Baily J, Love GD, Girkin JM, Mullins JJ, Tucker CS, Denvir MA (2013) Laser-targeted ablation of the zebrafish embryonic ventricle: a novel model of cardiac injury and repair. Int J Cardiol 168:3913–3919. https://doi.org/10.1016/j.ijcard.2013.06.063
Mikawa T, Borisov A, Brown AM, Fischman DA (1992) Clonal analysis of cardiac morphogenesis in the chicken embryo using a replication-defective retrovirus: I. Formation of the ventricular myocardium. Dev Dyn 193:11–23. https://doi.org/10.1002/aja.1001930104
Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT, Szweda LI, Xing C, Hu Z, Deberardinis RJ, Schiattarella G, Hill JA, Oz O, Lu Z, Zhang CC, Kimura W, Sadek HA (2017) Hypoxia induces heart regeneration in adult mice. Nature 541:222–227. https://doi.org/10.1038/nature20173
Nakajima Y (2019) Retinoic acid signaling in heart development. Genesis 57:e23300. https://doi.org/10.1002/dvg.23300
Palmquist-Gomes P, Guadix JA, Pérez-Pomares JM (2016) A chick embryo cryoinjury model for the study of embryonic organ development and repair. Differentiation 91:71–77. https://doi.org/10.1016/j.diff.2015.10.011
Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massagué J (1994) Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78:59–66. https://doi.org/10.1016/0092-8674(94)90572-x
Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA (2011) Transient regenerative potential of the neonatal mouse heart. Sci 331:1078–1080. https://doi.org/10.1126/science.1200708
Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, Mammen PP, Rothermel BA, Olson EN, Sadek HA (2013) Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci USA 110:187–192. https://doi.org/10.1073/pnas.1208863110
Poss KD, Wilson LG, Keating MT (2002) Heart regeneration in zebrafish. Sci 298:2188–2190. https://doi.org/10.1126/science.1077857
Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI, Sadek HA (2014) The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 157:565–579. https://doi.org/10.1016/j.cell.2014.03.032
Sedmera D, Thompson RP (2011) Myocyte proliferation in the developing heart. Dev Dyn 240:1322–1334. https://doi.org/10.1002/dvdy.22650
Sturzu AC, Rajarajan K, Passer D, Plonowska K, Riley A, Tan TC, Sharma A, Xu AF, Engels MC, Feistritzer R, Li G, Selig MK, Geissler R, Robertson KD, Scherrer-Crosbie M, Domian IJ, Wu SM (2015) Fetal mammalian heart generates a robust compensatory response to cell loss. Circulation 132:109–121. https://doi.org/10.1161/CIRCULATIONAHA.114.011490
van den Berg G, Abu-Issa R, de Boer BA, Hutson MR, de Boer PA, Soufan AT, Ruijter JM, Kirby ML, van den Hoff MJ, Moorman AF (2009) A caudal proliferating growth center contributes to both poles of the forming heart tube. Circ Res 104:179–188. https://doi.org/10.1161/circresaha.108.185843
Villa Del Campo C, Clavería C, Sierra R, Torres M (2014) Cell competition promotes phenotypically silent cardiomyocyte replacement in the mammalian heart. Cell Rep 8:1741–1751. https://doi.org/10.1016/j.celrep.2014.08.005
Witman N, Murtuza B, Davis B, Arner A, Morrison JI (2011) Recapitulation of developmental cardiogenesis governs the morphological and functional regeneration of adult newt hearts following injury. Dev Biol 354:67–76. https://doi.org/10.1016/j.ydbio.2011.03.021
Funding
Japan Society for the Promotion of Science (JSPS); Grant numbers: 25460273, 19K07251.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Animal handling and procedures were approved by the Osaka City University Animal Care and Use Committee (approval reference number 18026), as specified in the NIH Guide for the Care and Use of Laboratory Animals (Eighth Edition).
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Narematsu, M., Nakajima, Y. The early embryonic heart regenerates by compensation of proliferating residual cardiomyocytes after cryoinjury. Cell Tissue Res 384, 757–769 (2021). https://doi.org/10.1007/s00441-021-03431-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-021-03431-w