Abstract
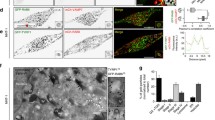
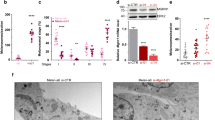
The melanosome, an organelle specialized for melanin synthesis, is one of the lysosome-related organelles. Its lumen is reported to be acidified by vacuolar-type H+-ATPase (V-ATPase). Mammalian V-ATPase exhibits structural diversity in its subunit isoforms; with regard to membrane intrinsic subunit a, four isoforms (a1–a4) have been found to be localized to distinct subcellular compartments. In this study, we have shown that the a3 isoform is co-localized with a melanosome marker protein, Pmel17, in mouse melanocytes. Acidotropic probes (LysoSensor and DAMP) accumulate in non-pigmented Pmel17-positive melanosomes, and DAMP accumulation is sensitive to bafilomycin A1, a specific inhibitor of V-ATPase. However, none of the subunit a isoforms is associated with highly pigmented mature melanosomes, in which the acidotropic probes are also not accumulated. oc/oc mice, which have a null mutation at the a3 locus, show no obvious defects in melanogenesis. In the mutant melanocytes, the expression of the a2 isoform is modestly elevated, and a considerable fraction of this isoform is localized to premature melanosomes. These observations suggest that the V-ATPase keeps the lumen of premature melanosomes acidic, whereas melanosomal acidification is less significant in mature melanosomes.










Similar content being viewed by others
References
Ancans J, Thody AJ (2000) Activation of melanogenesis by vacuolar type H+-ATPase inhibitors in amelanotic, tyrosinase positive human and mouse melanoma cells. FEBS Lett 478:57–60
Ancans J, Hoogduijn MJ, Thody AJ (2001a) Melanosomal pH, pink locus protein and their roles in melanogenesis. J Invest Dermatol 117:158–159
Ancans J, Tobin DJ, Hoogduijn MJ, Smit NP, Wakamatsu K, Thody AJ (2001b) Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Exp Cell Res 268:26–35
Basrur V, Yang F, Kushimoto T, Higashimoto Y, Yasumoto K, Valencia J, Muller J, Vieira WD, Watabe H, Shabanowitz J, Hearing VJ, Hunt DF, Appella E (2003) Proteomic analysis of early melanosomes: identification of novel melanosomal proteins. J Proteome Res 2:69–79
Beermann F, Orlow SJ, Lamoreux ML (2004) The Tyr (albino) locus of the laboratory mouse. Mamm Genome 15:749–758
Berson JF, Harper DC, Tenza D, Raposo G, Marks MS (2001) Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol Biol Cell 12:3451–3464
Bhatnagar V, Ramalah A (1998) Characterization of Mg2+-ATPase activity in isolated B16 murine melanoma melanosomes. Mol Cell Biochem 189:99–106
Bhatnagar V, Anjaiah S, Puri N, Darshanam BN, Ramaiah A (1993) pH of melanosomes of B 16 murine melanoma is acidic: its physiological importance in the regulation of melanin biosynthesis. Arch Biochem Biophys 307:183–192
Blott EJ, Griffiths GM (2002) Secretory lysosomes. Nat Rev Mol Cell Biol 3:122–131
Brilliant M, Gardner J (2001) Melanosomal pH, pink locus protein and their roles in melanogenesis. J Invest Dermatol 117:386–387
Chi A, Valencia JC, Hu ZZ, Watabe H, Yamaguchi H, Mangini NJ, Huang H, Canfield VA, Cheng KC, Yang F, Abe R, Yamagishi S, Shabanowitz J, Hearing VJ, Wu C, Appella E, Hunt DF (2006) Proteomic and bioinformatic characterization of the biogenesis and function of melanosomes. J Proteome Res 5:3135–3144
Costin GE, Valencia JC, Vieira WD, Lamoreux ML, Hearing VJ (2003) Tyrosinase processing and intracellular trafficking is disrupted in mouse primary melanocytes carrying the underwhite (uw) mutation. A model for oculocutaneous albinism (OCA) type 4. J Cell Sci 116:3203–3212
Devi CC, Tripathi RK, Ramaiah A (1987) pH-dependent interconvertible allosteric forms of murine melanoma tyrosinase. Physiological implications. Eur J Biochem 166:705–711
Di Pietro SM, Dell’Angelica EC (2005) The cell biology of Hermansky-Pudlak syndrome: recent advances. Traffic 6:525–533
Dickie MM (1967) Osteosclerotic (oc). Mouse News Lett 36:39
Diment S, Eidelman M, Rodriguez GM, Orlow SJ (1995) Lysosomal hydrolases are present in melanosomes and are elevated in melanizing cells. J Biol Chem 270:4213–4215
Donatien PD, Orlow SJ (1995) Interaction of melanosomal proteins with melanin. Eur J Biochem 232:159–164
Futai M, Oka T, Moriyama Y, Wada Y (1998) Diverse roles of single membrane organelles: factors establishing the acid lumenal pH. J Biochem 124:259–267
Gu F, Gruenberg J (2000) ARF1 regulates pH-dependent COP functions in the early endocytic pathway. J Biol Chem 275:8154–8160
Halaban R, Patton RS, Cheng E, Svedine S, Trombetta ES, Wahl ML, Ariyan S, Hebert DN (2002) Abnormal acidification of melanoma cells induces tyrosinase retention in the early secretory pathway. J Biol Chem 277:14821–14828
Hearing VJ, Ekel TM (1976) Mammalian tyrosinase. A comparison of tyrosine hydroxylation and melanin formation. Biochem J 157:549–557
Hurtado-Lorenzo A, Skinner M, El Annan J, Futai M, Sun-Wada GH, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA, Brown D, Marshansky V (2006) V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol 8:124–136
Kornfeld S, Mellman I (1989) The biogenesis of lysosomes. Annu Rev Cell Biol 5:483–525
Manga P, Orlow SJ (2001) Inverse correlation between pink-eyed dilution protein expression and induction of melanogenesis by bafilomycin A1. Pigment Cell Res 14:362–367
Mani I, Sharma V, Tamboli I, Raman G (2001) Interaction of melanin with proteins—the importance of an acidic intramelanosomal pH. Pigment Cell Res 14:170–179
Matsuo H, Chevallier J, Mayran N, Le Blanc I, Ferguson C, Faure J, Blanc NS, Matile S, Dubochet J, Sadoul R, Parton RG, Vilbois F, Gruenberg J (2004) Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 303:531–534
Mellman I, Fuchs R, Helenius A (1986) Acidification of the endocytic and exocytic pathways. Annu Rev Biochem 55:663–700
Murata Y, Sun-Wada GH, Yoshimizu T, Yamamoto A, Wada Y, Futai M (2002) Differential localization of the vacuolar H+ pump with G subunit isoforms (G1 and G2) in mouse neurons. J Biol Chem 277:36296–36303
Nguyen T, Novak EK, Kermani M, Fluhr J, Peters LL, Swank RT, Wei ML (2002) Melanosome morphologies in murine models of Hermansky-Pudlak syndrome reflect blocks in organelle development. J Invest Dermatol 119:1156–1164
Oikawa A, Saeki H, Akiyama T, Matsumoto J (1987) Electron microscopic evidence for stimulation of melanosomal maturation by lysosomotropic agents and monensin in cultured B16 mouse melanoma cells. Pigment Cell Res 1:44–50
Oka T, Murata Y, Namba M, Yoshimizu T, Toyomura T, Yamamoto A, Sun-Wada GH, Hamasaki N, Wada Y, Futai M (2001) a4, a unique kidney-specific isoform of mouse vacuolar H+-ATPase subunit a. J Biol Chem 276:40050–40054
Orlow SJ (1995) Melanosomes are specialized members of the lysosomal lineage of organelles. J Invest Dermatol 105:3–7
Pietrement C, Sun-Wada GH, Silva ND, McKee M, Marshansky V, Brown D, Futai M, Breton S (2006) Distinct expression patterns of different subunit isoforms of the V-ATPase in the rat epididymis. Biol Reprod 74:185–194
Puri N, Gardner JM, Brilliant MH (2000) Aberrant pH of melanosomes in pink-eyed dilution (p) mutant melanocytes. J Invest Dermatol 115:607–613
Raposo G, Marks MS (2002) The dark side of lysosome-related organelles: specialization of the endocytic pathway for melanosome biogenesis. Traffic 3:237–248
Raposo G, Tenza D, Murphy DM, Berson JF, Marks MS (2001) Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J Cell Biol 152:809–824
Saeki H, Oikawa A (1983) Stimulation of tyrosinase activity of cultured melanoma cells by lysosomotropic agents. J Cell Physiol 116:93–97
Saeki H, Oikawa A (1985) Stimulation by ionophores of tyrosinase activity of mouse melanoma cells in culture. J Invest Dermatol 85:423–425
Scimeca JC, Franchi A, Trojani C, Parrinello H, Grosgeorge J, Robert C, Jaillon O, Poirier C, Gaudray P, Carle GF (2000) The gene encoding the mouse homologue of the human osteoclast-specific 116-kDa V-ATPase subunit bears a deletion in osteosclerotic (oc/oc) mutants. Bone 26:207–213
Smith AN, Skaug J, Choate KA, Nayir A, Bakkaloglu A, Ozen S, Hulton SA, Sanjad SA, Al-Sabban EA, Lifton RP, Scherer SW, Karet FE (2000) Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat Genet 26:71–75
Stevens TH, Forgac M (1997) Structure, function and regulation of the vacuolar (H+)-ATPase. Annu Rev Cell Dev Biol 13:779–808
Strauss O (2005) The retinal pigment epithelium in visual function. Physiol Rev 85:845–881
Sun-Wada GH, Murata Y, Yamamoto A, Kanazawa H, Wada Y, Futai M (2000) Acidic endomembrane organelles are required for mouse postimplantation development. Dev Biol 228:315–325
Sun-Wada GH, Imai-Senga Y, Yamamoto A, Murata Y, Hirata T, Wada Y, Futai M (2002) A proton pump ATPase with testis-specific E1 subunit isoform required for acrosome acidification. J Biol Chem 277:18098–18105
Sun-Wada GH, Murata Y, Namba M, Yamamoto A, Wada Y, Futai M (2003a) Mouse proton pump ATPase C subunit isoforms (C2-a and C2-b) specifically expressed in kidney and lung. J Biol Chem 278:44843–44851
Sun-Wada GH, Wada Y, Futai M (2003b) Lysosome and lysosome-related organelles responsible for specialized functions in higher organisms, with special emphasis on vacuolar-type proton ATPase. Cell Struct Funct 28:455–463
Sun-Wada GH, Yoshimizu T, Imai-Senga Y, Wada Y, Futai M (2003c) Diversity of mouse proton-translocating ATPase: presence of multiple isoforms of the C, d and G subunits. Gene 302:147–153
Sun-Wada GH, Toyomura T, Murata Y, Yamamoto A, Futai M, Wada Y (2006) The a3 isoform of V-ATPase regulates insulin secretion from pancreatic β-cells. J Cell Sci 119:4531–4540
Sun-Wada GH, Tabata H, Kawamura N, Futai M, Wada Y (2007) Differential expression of a subunit isoforms of the vacuolar-type proton pump ATPase in mouse endocrine tissues. Cell Tissue Res 329:239–248
Swank RT, Novak EK, McGarry MP, Rusiniak ME, Feng L (1998) Mouse models of Hermansky Pudlak syndrome. Pigment Cell Res 11:60–80
Theos AC, Truschel ST, Raposo G, Marks MS (2005) The silver locus product Pmel17/gp100/Silv/ME20: controversial in name and in function. Pigment Cell Res 18:322–336
Toyomura T, Oka T, Yamaguchi C, Wada Y, Futai M (2000) Three subunit a isoforms of mouse vacuolar H+-ATPase. Preferential expression of the a3 isoform during osteoclast differentiation. J Biol Chem 275:8760–8765
Toyomura T, Murata Y, Yamamoto A, Oka T, Sun-Wada GH, Wada Y, Futai M (2003) From lysosomes to plasma membrane: localization of vacuolar type H+-ATPase with the a3 isoform during osteoclast differentiation. J Biol Chem 278:22023–22030
Tripathi RK, Chaya Devi C, Ramaiah A (1988) pH-dependent interconversion of two forms of tyrosinase in human skin. Biochem J 252:481–487
Watabe H, Valencia JC, Yasumoto K, Kushimoto T, Ando H, Muller J, Vieira WD, Mizoguchi M, Appella E, Hearing VJ (2004) Regulation of tyrosinase processing and trafficking by organellar pH and by proteasome activity. J Biol Chem 279:7971–7981
Yasumoto K, Watabe H, Valencia JC, Kushimoto T, Kobayashi T, Appella E, Hearing VJ (2004) Epitope mapping of the melanosomal matrix protein gp100 (PMEL17): rapid processing in the endoplasmic reticulum and glycosylation in the early Golgi compartment. J Biol Chem 279:28330–28338
Author information
Authors and Affiliations
Corresponding author
Additional information
Ge-Hong Sun-Wada and Yoh Wada contributed equally to this study.
This study was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by the Hayashi and Noda Foundations.
Rights and permissions
About this article
Cite this article
Tabata, H., Kawamura, N., Sun-Wada, GH. et al. Vacuolar-type H+-ATPase with the a3 isoform is the proton pump on premature melanosomes. Cell Tissue Res 332, 447–460 (2008). https://doi.org/10.1007/s00441-008-0597-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-008-0597-5