Abstract
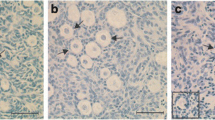
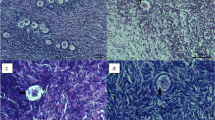
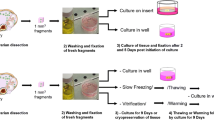
Caprine preantral follicles within ovarian fragments were cryopreserved in the absence or presence of 0.5 M sucrose with or without 1 M dimethyl sulfoxide and/or 1 M ethylene glycol (EG). After being thawed, they were washed in minimum essential medium with or without 0.3 M sucrose. Histological analysis of follicle integrity immediately after cryopreservation showed consistent beneficial effects of including sucrose in the three cryoprotectant solutions analyzed when tissue was thawed without sucrose (53.9±14.8–82.4±3.2% normal vs 27.6±1.6–36.6±6.5%, P<0.05). However, in further studies, the addition of sucrose to the thaw solutions proved detrimental or of no benefit. An analysis of the cryopreserved material with calcein-AM and ethidium homodimer (markers for living and dead cells, respectively) gave comparable results to those obtained by histology. Follicles cryopreserved in EG, EG plus sucrose, or sucrose alone were cultured in vitro for 24 h following warming. During this culture period, viability fell most rapidly in material cryopreserved in sucrose alone and was no longer correlated with either the viability or integrity estimates made immediately after warming. By contrast, the viability of follicles cryopreserved in EG with sucrose and then cultured for 24 h was not significantly different from the cultured non-frozen controls. These results indicate that cryopreservation in 1 M EG plus 0.5 M sucrose combined with thawing without sucrose is effective for caprine ovarian tissue.





Similar content being viewed by others
References
Amorim CA, Rodrigues APR, Rondina D, Goncalves PBD, Figueiredo JR, Giorgetti A (2003a) Cryopreservation of ovine primordial follicles using dimethyl sulfoxide. Fertil Steril 79:682–686
Amorim CA, Rondina D, Rodrigues APR, Costa SHF, Goncalves PBD, Figueiredo JR, Giorgetti A (2003b) Isolated ovine primordial follicles cryopreserved in different concentrations of ethylene glycol. Theriogenology 60:735–742
Amorim CA, Rondina D, Rodrigues APR, Goncalves PBD, Figueiredo JR, Giorgetti A (2004) Cryopreservation of isolated ovine primordial follicles with propylene glycol and glycerol. Fertil Steril 81:735–740
Bianchi V, Coticchio G, Fava L, Flamigni C, Borini A (2005) Meiotic spindle imaging in human oocytes frozen with a slow freezing procedure involving high sucrose concentration. Hum Reprod 20:1078–1083
Capacchietti G, Cecconi S, Gioia L, Turriani M (2004) Effect of cryoprotectant agents on the potential development of sheep preantral follicles. Vet Res Commun 28:173–176
Cecconi S, Capacchietti G, Russo V, Berardinelli P, Mattioli M, Barboni B (2004) In vitro growth of preantral follicles isolated from cryopreserved ovine ovarian tissue. Biol Reprod 70:12–17
Chen ZJ, Li M, Li Y, Zhao LX, Tang R, Sheng Y, Gao X, Chang CH, Feng HL (2004) Effects of sucrose concentration on the developmental potential of human frozen-thawed oocytes at different stages of maturity. Hum Reprod 19:2345–2349
Cortvrindt RG, Smitz JEJ (2001) Fluorescent probes allow rapid and precise recording of follicle density and staging in human ovarian cortical biopsy samples. Fertil Steril 75:588–593
De Clerck LS, Bridts CH, Mertens AM, Moens MM, Stevens WJ (1994) Use of fluorescent dyes in the determination of adherence of human leucocytes to endothelial cells and the effect of fluorochromes on cellular function. J Immunol Methods 172:115–124
Demirci B, Salle B, Frappart L, Franck M, Guerin JF, Lornage J (2002) Morphological alterations and DNA fragmentation in oocytes from primordial and primary follicles after freezing-thawing of ovarian cortex in sheep. Fertil Steril 77:595–600
Devireddy RV (2005) Predicted permeability parameters of human ovarian tissue cells to various cryoprotectants and water. Mol Reprod Dev 70:333–343
Devireddy RV, Bischof JC (2003) Recent advances in cryobiology using calorimetry. In: Kakac S, Smirnov H, Mila MR (ed) Low temperature and cryogenic refrigeration. Kluwer, The Netherlands, pp 265–294
Fabri R, Porcu E, Marsella T, Rocchetta G, Venturoli S, Flamigni C (2001) Human oocyte cryopreservation: new perspectives regarding oocyte survival. Hum Reprod 16:411–416
Gook DA, Edgar DH, Borg J, Archer J, McBain JC (2005) Diagnostic assessment of the development potential of human cryopreserved ovarian tissue from multiple patients using xenografting. Hum Reprod 20:72–78
Hovatta O, Silye R, Krausz T, Abir R, Margara R, Trew G, Lass A, Winston RM (1996) Cryopreservation of human ovarian tissue using dimethylsulphoxide and propanediol-sucrose as cryoprotectants. Hum Reprod 11:1268–1272
Hulshof SCJ, Figueiredo JR, Beckers JF, Bevers MM, Van Den Donk HA, Van Den Hurk R (1995) Effects of fetal bovine serum, FSH and 17 β-estradiol on the culture of bovine preantral follicles. Theriogenology 44:217–226
Jorio A, Mariana JC, Lahlou-Kassi A (1991) Development of the population of ovarian follicles during the prepubertal period in D’man and Timahdite sheep. Anim Reprod Sci 26:239–250
Liu RH, Sun QY, Li YH, Jiao LH, Wang WH (2003) Maturation of porcine oocytes after cooling at the germinal vesicle stage. Zygote 11:299–305
Lucci CM, Amorim CA, Bao SN, Figueiredo JR, Rodrigues APR, Silva JRV, Goncalves PBD (1999) Effect of the interval of serial sections of ovarian tissue in the tissue chopper on the number of isolated caprine preantral follicles. Anim Reprod Sci 56:39–49
Lucci CM, Kacinskis MA, Lopes LHR, Rumpf R, Bao SN (2004) Effect of different cryoprotectants on the structural preservation of follicles in frozen zebu bovine (Bos indicus) ovarian tissue. Theriogenology 61:1101–1114
Mandelbaum J, Junca AM, Plachot M, Alnot MO, Alvarez S, Debache C, Salat-Baroux J, Cohen J (1988) Human embryo cryopreservation, extrinsic and intrinsic parameters of success. Hum Reprod 2:709–715
Martinez-Madrid B, Dolmans MM, Van Langendonckt A, Defrere S, Donnez J (2004) Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril 82:1390–1394
Newton H, Illingworth P (2001) In vitro growth of murine preantral follicles after isolation from cryopreserved ovarian tissue. Hum Reprod 16:423–429
Newton H, Fisher J, Arnold JRP, Pegg DE, Faddy MJ, Gosden RG (1998) Permeation of human ovarian tissue with cryoprotective agents in preparation for cryopreservation. Hum Reprod 13:376–380
Nowshari MA, Ali SA, Saleem S (2005) Offspring resulting from transfer of cryopreserved embryos in camel (Camelus dromedarius). Theriogenology 63:2513–2522
Pedro PB, Yokoyama E, Zhu SE, Yoshida N, Valdez DM Jr, Tanaka M, Edashige K, Kasai M (2005) Permeability of mouse oocytes and embryos at various developmental stages to five cryoprotectants. J Reprod Dev 51:235–246
Poole CA, Brookes NH, Clover GM (1993) Keratocyte networks visualized in the living cornea using vital dyes. J Cell Sci 106:685–692
Rodrigues APR, Amorim CA, Costa SH, Matos MHT, Santos RR, Lucci CM, Bao SN, Ohashi OM, Figueiredo JR (2004a) Cryopreservation of caprine ovarian tissue using glycerol and ethylene glycol. Theriogenology 61:1009–1024
Rodrigues APR, Amorim CA, Costa SH, Matos MHT, Santos RR, Lucci CM, Bao SN, Ohashi OM, Figueiredo JR (2004b) Cryopreservation of caprine ovarian tissue using dimethylsulphoxide and propanediol. Anim Reprod Sci 84:211–227
Rodrigues APR, Amorim CA, Costa SHF, Santos RR, Lucci CM, JF Nunes, Figueiredo JR (2005) Cryopreservation and short-term culture of isolated caprine primordial follicles. J Small Rum Res 56:103–111
Salehnia M (2002) Autograft of vitrified mouse ovaries using ethylene glycol as cryoprotectant. Exp Anim 51:509–512
Santos RR, Rodrigues APR, Costa SHF, Silva JRV, Matos MHT, Lucci CM, Bao SN, Van den Hurk R, Figueiredo JR (2006) Histological and ultrastructural analysis of cryopreserved sheep preantral follicles. Anim Reprod Sci 91:249–263
Schotanus K, Hage WJ, Vanderstchele H, Van den Hurk R (1997) Effects of conditioned media from murine granulosa cell lines on the growth of isolated bovine preantral follicles. Theriogenology 48:471–483
Smith GD, Silva E, Silva CA (2004) Developmental consequences of cryopreservation of mammalian oocytes and embryos. Reprod Biomed Online 9:171–178
Van den Hurk R, Spek ER, Hage WJ, Fair T, Ralph JH, Schotanus K (1998) Ultrastructure and viability of isolated bovine preantral follicles. Hum Reprod Up 4:833–841
Acknowledgements
The authors thank Peter Ursem, Arend Rijneveld, Ineke Daemen, Frans van Kooi, and the technicians from the Department of Pathobiology for their help.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by CAPES/Brazil. Regiane Rodrigues dos Santos is a recipient of a grant from FUNCAP of Brazil.
Rights and permissions
About this article
Cite this article
Santos, R.R., Tharasanit, T., Figueiredo, J.R. et al. Preservation of caprine preantral follicle viability after cryopreservation in sucrose and ethylene glycol. Cell Tissue Res 325, 523–531 (2006). https://doi.org/10.1007/s00441-006-0193-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-006-0193-5