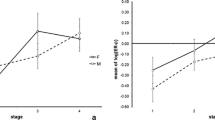
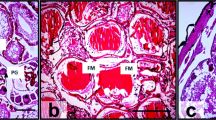
Abstract
Estrogen is involved in regulating the development and hormone secretion of the anterior pituitary gland following its binding to estrogen receptors (ERs) expressed on pituitary cells. However, the pituitary is comprised of several cell types, and to date, there is no data about the specific cell types expressing ERs in embyonic chick pituitary. We therefore followed, by immunohistochemistry, the ontogeny of the pituitary ER alpha (ERα), and the cell types expressing ERα throughout chick embryo development. ERα immunoreacitivity was restricted to the nuclei of pituitary cells. ERα-immunopositive (ERα+) cells were first detected at embryonic day 6.5 (E6.5), after which ERα+ cells were consistently detected throughout the anterior pituitary gland, although the density of ERα+ cells in the caudal lobe of the pars distalis was higher than that in the cephalic lobe. The proportion of ERα+ cells in the pituitary was about 6% at E8.5; expression increased to 22% by E18.5 of gestation, with no additional change until hatching. Double-labeling of ERα and pituitary hormones showed that the dominant cell types expressing ERα were gonadotrophs immunopositive for luteinizing hormone (LH); the proportion of ERα+ cells expressing LH increased throughout gestation and reached approximately 57% at hatching. About 2%–6% of thyroid-stimulating-hormone-immunopositive and 1%–2% prolactin-immunopositive cells expressed ERα at later stages of embryonic development, but no growth-hormone-positive or adrenocorticotropic-hormone-positive cells expressed ERα during the embryonic period. Thus, gonadotrophs are the main cell population expressing ERα in the anterior pituitary gland of chick embryo, and ERα is involved in regulating the development of the pituitary gland and the maturation of the hormone-secreting function.




Similar content being viewed by others
References
Baratta M, West LA, Turzillo AM, Nett TM (2001) Activin modulates differential effects of estradiol on synthesis and secretion of follicle-stimulating hormone in ovine pituitary cells. Biol Reprod 64:714–719
Bethea CL (1984) Stimulatory effect of estrogen on prolactin secretion from primate pituitary cells cultured on extracellular matrix and in serum-free medium. Endocrinology 115:443–451
Brandenberger AW, Tee MK, Lee JY, Chao V, Jaffe RB (1997) Tissue distribution of estrogen receptors alpha (ER-alpha) and beta (ER-beta) mRNA in the midgestational human fetus. J Clin Endocrinol Metab 82:3509–3512
Couse JF, Korach KS (1999) Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417
Cui S, Goldstein RS (2000) Expression of estrogen receptors in the dorsal root ganglia of the chick embryo. Brain Res 882:236–240
Cui S, Liu JL, Shao YJ, Zhang JC (2004) Parallel changes between the percentage of fetal pituitary cells immunoreactive to oestrogen receptor α and the concentration of 17β-oestradiol in fetal and maternal plasma during gestation in sheep. Reprod Fert Dev 16:611–616
Demay F, Tiffoche C, Thieulant ML (1996) Sex- and cell-specific expression of an estrogen receptor isoform in the pituitary gland. Neuroendocrinology 63:522–529
Friend KE, Chiou YK, Lopes MBS, Laws ER, Hughes JKM, Shupnik MA (1994) Estrogen receptor expression in human pituitary: correlation with immunohistochemistry in normal tissue, and immunohistochemistry and morphology in macroadenomas. J Clin Endocrinol Metab 78:1497–1504
Friend KE, Ang LW, Shupnik MA (1995) Estrogen regulates the expression of several different estrogen receptor mRNA isoforms in rat pituitary. Proc Natl Acad Sci USA 92:4367–4371
Friend KE, Resnick EM, Ang LW, Shupnik MA (1997) Specific modulation of estrogen receptor mRNA isoforms in rat pituitary throughout the estrous cycle and in response to steroid hormones. Mol Cell Endocrinol 131:147–155
Gasc JM, Sar M, Stumpf WE (1980) Immunocharacteristics of oestrogen and androgen target cells in the anterior pituitary gland of the chick embryo as demonstrated by a combined method of autoradiography and immunohistochemistry. J Endocrinol 86:245–250
Griffin C, Flouriot G, Sonntag-Buck V, Nestor P, Gannon F (1998) Identification of novel chicken estrogen receptor-alpha mRNA isoforms generated by alternative splicing and promoter usage. Endocrinology 139:4614–4625
Griffin C, Flouriot G, Sonntag-Buck V, Gannon F (1999) Two functionally different protein isoforms are produced from the chicken estrogen receptor-alpha gene. Mol Endocrinol 13:1571–1587
Griffin C, Flouriot G, Sharp P, Greene G, Gannon F (2001) Distribution analysis of the two chicken estrogen receptor-alpha isoforms and their transcripts in the hypothalamus and anterior pituitary gland. Biol Reprod 65:1156–1163
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–92
Hashi A, Mazawa S, Chen SY, Yamakawa K, Kato J, Arita J (1996) 17β-Estradiol-induced diurnal changes in lactotroph proliferation and their hypothalamic regulation in ovariectomized rats. Endocrinology 137:3246–3252
Keefer DA, Stumpf WE, Petrusz P (1976) Quantitative autoradiographic assessment of 3H-estradiol uptake in immunocytochemically characterized pituitary cells. Cell Tissue Res 166:25–35
King JA, Davidson JS, Mehl AEI, Wakefield IK, Andersson PB, Millar RP (1989) Gonadal steroid modulation of signal transduction and luteinizing hormone release in cultured chicken pituitary cells. Endocrinology 124:1830–1840
Krust A, Green S, Argos P, Kumar V, Walter P, Bornert JM, Chamborn P (1986) The chicken oestrogen receptor sequence: homology with v-erbA and the human oestrogen and glucocorticoid receptors. EMBO J 5:891–897
Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA (1996) Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930
Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA (1997) Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138:863–870
Lakaye B, Foidart A, Grisar T, Balthazart J (1998) Partial cloning and distribution of estrogen receptor beta in the avian brain. Neuroreport 9:2743–2748
Lea RW, Richard-Yris MA, Sharp PJ (1996) The effect of ovariectomy on concentrations of plasma prolactin and LH and parental behavior in the domestic fowl. Gen Comp Endocrinol 101:115–121
Mikami S (1983) Avian adenohypophysis: recent progress in immunocytochemical studies. In: Mikami S, Homma K, Wada M (eds) Avian endocrinology: environmental and ecological perspectives. Japan Sci Soc, Tokyo, pp 39–56
Mitchner NA, Garlick C, Ben-Jonathan N (1998) Cellular distribution and gene regulation of estrogen receptors α and β in the rat pituitary gland. Endocrinology 139:3976–3983
Molter-Gerard C, Caraty A, Guerin S, Fontaine J, Taragnat C (2000) Dynamic changes in the gonadotrope cell populations during an estradiol-induced surge in the ewe. Biol Reprod 63:1084–1091
Mosselman S, Polman J, Dijkema R (1996) ER-β: identification and characterization of a novel human estrogen receptor. FEBS Lett 392:49–53
Murphy AE, Harvey S (2001) Extrapituitary βTSH and GH in early chick embryos. Mol Cell Endocrinol 185:161–171
Nishihara E, Nagayama Y, Inoue S, Hiroi H, Muramatsu M, Yamashita S, Koji T (2000) Ontogenetic changes in the expression of estrogen receptor alpha and beta in rat pituitary gland detected by immunohistochemistry. Endocrinology 141:615–620
Oomizu S, Honda J, Takeuchi S, Kakeya T, Masui T, Takahashi S (2000) Transforming growth factor-alpha stimulates proliferation of mammotrophs and corticotrophs in the mouse pituitary. J Endocrinol 165:493–501
Pasqualini C, Guivarc’h D, Boxberg YV, Nothias F, Vincent JD, Vernier P (1999) Stage- and region-specific expression of estrogen receptor alpha isoforms during ontogeny of the pituitary gland. Endocrinology 140:2781–2789
Proudman JA, Vandesande F, Berghman LR (1999) Immunohistochemical evidence that follicle-stimulating hormone and luteinizing hormone reside in separate cells in the chicken pituitary. Biol Reprod 60:1324–1328
Puebla-Osorio N, Proudman JA, Compton AE, Clements KE, Decuypere E, Vandesande F, Berghman LR (2002) FSH- and LH-cells originate as separate cell populations and at different embryonic stages in the chicken embryo. Gen Comp Endocrinol 127:242–248
Rombauts L, Berghman LR, Vanmontfort D, Decuypere E, Verhoeven G (1993) Changes in immunoreactive FSH and inhibin in developing chicken embryos and the effects of estradiol and the aromatase inhibitor R76713. Biol Reprod 49:549–554
Sasaki F, Doshita A, Matsumoto Y, Kuwahara S, Tsukamoto Y, Ogawa K (2003) Embryonic development of the pituitary gland in the chick. Cells Tissues Organs 173:65–74
Saunders PTK (1998) Oestrogen receptor beta (ER-beta). Rev Reprod 3:164–171
Scanlan N, Skinner DC (2002) Estradiol modulation of growth hormone secretion in the ewe: no growth hormone-releasing hormone neurons and few somatotropes express estradiol receptor alpha. Biol Reprod 66:1267–1273
Schreihofer DA, Stoler MH, Shupnik MA (2000) Differential expression and regulation of estrogen receptors (ERs) in rat pituitary and cell lines: estrogen decreases ER alpha protein and estrogen responsiveness. Endocrinology 141:2174–2184
Scully KM, Gleiberman AS, Lindzey J, Lubahn DB, Korach KS, Rosenfeld MG (1997) Role of estrogen receptor-α in the anterior pituitary gland. Mol Endocrinol 11:674–681
Sheng C, McNeilly AS, Brooks AN (1998) Immunohistochemical distribution of oestrogen receptor and luteinizing hormone B subunit in the ovine pituitary gland during foetal development. J Neuroendocrinol 10:713–718
Shupnik MA, Rosenzweig BA (1991) Identification of an estrogen-responsive element in the rat LH beta gene. DNA-estrogen receptor interactions and functional analysis. J Biol Chem 266:17084–17091
Shupnik MA, Gharib SD, Chin WW (1988) Estrogen suppresses rat gonadotropin gene transcription in vivo. Endocrinology 122:1842–1846
Shupnik MA, Gharib SD, Chin WW (1989) Divergent effects of estradiol on gonadotropin gene transcription in pituitary fragments. Mol Endocrinol 3:474–480
Stefaneanu L, Kovacs K, Horvath E, Lloyd RV, Buchfelder M, Fahlbusch R, Smyth H (1994) In situ hybridization study of estrogen receptor messenger ribonucleic acid in human adenohypophysial cells and pituitary adenomas. J Clin Endocrinol Metab 78:83–88
Tobin VA, Pompolo S, Clarke IJ (2001) The percentage of pituitary gonadotropes with immunoreactive oestradiol receptors increases in the follicular phase of the ovine oestrous cycle. J Neuroendocrinol 13:846–854
Watters JJ, Chun TY, Kim YN, Bertics PJ, Gorski J (2000) Estrogen modulation of prolactin gene expression requires an intact mitogen-activated protein kinase signal transduction pathway in cultured rat pituitary cells. Mol Endocrinol 14:1872–1881
Wilson ME, Price RH Jr, Handa RJ (1998) Estrogen receptor-beta messenger ribonucleic acid expression in the pituitary gland. Endocrinology 139:5151–5156
Woods JE, Brazzill DM (1981) Plasma 17 β-estradiol levels in the chick embryo. Gen Comp Endocrinol 44:37–43
Woods JE, Otten LM, Thommes RC (1995) Ontogeny of 17B-estradiol (E2) and estrogen receptor (ER)-immunostained cells in the hypothalamus and adenohyophyseal pars distalis of the chick embryo. Growth Dev Aging 59:93–105
Zafar M, Ezzat S, Ramyar L, Pan N, Smyth HS, Asa SL (1995) Cell-specific expression of estrogen receptor in the human pituitary and its adenomas. J Clin Endocrinol Metab 80:3621–3627
Acknowledgements
We thank Prof. P. Sharp (Roslin Institute, UK) for the kind gift of polyclonal anti-chicken PRL primary antibody, and Dr. T. Matozaki (Gunma University, Japan) for the kind gift of polyclonal anti-chicken LH primary antibody. Anti-chicken GH, anti-rat TSH, and anti-human ACTH primary antibodies were obtained through the National Hormone and Peptide Program (NHPP), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and Dr. Parlow (NHPP). We also thank Dr. L. R. Berghman (Texas A&M University) for the kind gift of monoclonal anti-chicken LH primary antibody, and Dr. J.A. Proudman (US Department of Agriculture) for help and suggestions. We are also grateful to Professor R. S. Goldstein, Faculty of Life Science, Bar-Ilan University, Israel, for reviewing the English manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from the Natural Science Foundation for Outstanding Young Scientists of China (30325034) and the Natural Science Foundation of China (30170693, 30471264).
Rights and permissions
About this article
Cite this article
Liu, J., Cui, S. Ontogeny of estrogen receptor (ER) alpha and its co-localization with pituitary hormones in the pituitary gland of chick embryos. Cell Tissue Res 320, 235–242 (2005). https://doi.org/10.1007/s00441-004-1051-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-1051-y