Abstract
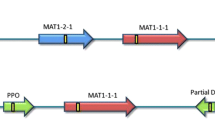
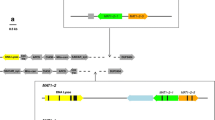
The genus Septoria contains more than 1000 species of plant pathogenic fungi, most of which have no known sexual stage. Species of Septoria without a known sexual stage could be recent derivatives of sexual species that have lost the ability to mate. To test this hypothesis, the mating-type region of S. passerinii, a species with no known sexual stage, was cloned, sequenced, and compared to that of its close relative S. tritici (sexual stage: Mycosphaerella graminicola). Both of the S. passerinii mating-type idiomorphs were approximately 3 kb in size and contained a single reading frame interrupted by one (MAT-2) or two (MAT-1) putative introns. The putative products of MAT-1 and MAT-2 are characterized by alpha-box and high-mobility-group sequences, respectively, similar to those in the mating-type genes of M. graminicola and other fungi. The mating-type genes of S. passerinii and M. graminicola are evolving rapidly, approximately ten times faster than the internal transcribed spacer region of the ribosomal DNA, and are not closely related to those from Cochliobolus or other loculoascomycetes in the order Pleosporales. Therefore, the class Loculoascomycetes may be polyphyletic. Furthermore, differences between the phylogenetic trees may indicate separate evolutionary histories for the MAT-1 and MAT-2 idiomorphs. A three-primer multiplex-PCR technique was developed that allowed rapid identification of the mating types of isolates of S. passerinii. Both mating types were present in approximately equal frequencies and often on the same leaf in fields in Minnesota and North Dakota. Analyses with isozyme and random amplified polymorphic DNA markers revealed that each isolate had a unique genotype. The common occurrence of both mating types on the same leaf and the high levels of genotypic diversity indicate that S. passerinii is almost certainly not an asexual derivative of a sexual fungus. Instead, sexual reproduction probably plays an integral role in the life cycle of S. passerinii and may be much more important than previously believed in this (and possibly other) "asexual" species of Septoria.





Similar content being viewed by others
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Barr ME (1979) A classification of the Loculoascomycetes. Mycologia 71:935–957
Berbee ML (2001) The phylogeny of plant and animal pathogens in the Ascomycota. Physiol Mol Plant Pathol 59:165–187
Berbee ML, Pirseyedi M, Hubbard S (1999) Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 91:964–977
Biel SW, Parrish FW (1986) Isolation of DNA from fungal mycelia and sclerotia without use of density gradient ultracentrifugation. Anal Biochem 154:21–25
Bruchez JJP, Eberle J, Russo VEA (1993) Regulatory sequences in the transcription of Neurospora crassa genes: CAAT box, TATA box, introns, poly(A) tail formation sequences. Fungal Genet Newslett 40:89–96
Christiansen SK, Wirsel S, Yun S-H, Yoder OC, Turgeon BG (1998) The two Cochliobolus mating type genes are conserved among species but one of them is missing in C. victoriae. Mycol Res 102:919–929
Coppin E, Debuchy R, Arnaise S, Picard M (1997) Mating types and sexual development in filamentous Ascomycetes. Microbiol Mol Biol Rev 61:411–428
Corlett M (1991) An annotated list of the published names in Mycosphaerella and Sphaerella. Mycol Mem 18:1–328
Cunfer BM, Ueng PP (1999) Taxonomy and identification of Septoria and Stagonospora species on small-grain cereals. Annu Rev Phytopathol 37:267–284
Dyer PS, Furneaux PA, Douhan G, Murray TD (2001) A multiplex PCR test for determination of mating type applied to the plant pathogens Tapesia yallundae and Tapesia acuformis. Fungal Genet Biol 33:173–180
Eriksson OE, Winka K (1998) Families and higher taxa of Ascomycota. Myconet 1:17–24
Foster SJ, Ashby AM, Fitt BDL (2002) Improved PCR-based assays for pre-symptomatic diagnosis of light leaf spot and determination of mating type of Pyrenopeziza brassicae on winter oilseed rape. Eur J Plant Pathol 108:379–383
Goodwin SB, Zismann VL (2001) Phylogenetic analyses of the ITS region of ribosomal DNA reveal that Septoria passerinii from barley is closely related to the wheat pathogen Mycosphaerella graminicola. Mycologia 93:934–946
Goodwin SB, Saghai Maroof MA, Allard RW, Webster RK (1993) Isozyme variation within and among populations of Rhynchosporium secalis in Europe, Australia and the United States. Mycol Res 97:49–58
Goodwin SB, Schneider RE, Fry WE (1995) Use of cellulose-acetate electrophoresis for rapid identification of allozyme genotypes of Phytophthora infestans. Plant Dis 79:1181–1185
Goodwin SB, Cavaletto JR, Waalwijk C, Kema GHJ (2001a) DNA fingerprint probe from Mycosphaerella graminicola identifies an active transposable element. Phytopathology 91:1181–1188
Goodwin SB, Dunkle LD, Zismann VL (2001b) Phylogenetic analysis of Cercospora and Mycosphaerella based on the internal transcribed spacer region of ribosomal DNA. Phytopathology 91:648–658
Green GJ, Dickson JG (1957) Pathological histology and varietal reactions in septoria leaf blotch of barley. Phytopathology 47:73–79
Hawksworth DL, Kirk PM, Sutton BC, Pegler DN (1995) Ainsworth and Bisby's Dictionary of the fungi. CAB International, Wallingford, UK
Hebert PDN, Beaton MJ (1993) Methodologies for allozyme analysis using cellulose acetate electrophoresis. Helena Laboratories, Beaumont, Tex. 32 pp
Hunter T, Coker RR, Royle DJ (1999) The teleomorph stage, Mycosphaerella graminicola, in epidemics of Septoria tritici blotch on winter wheat in the UK. Plant Pathol 48:51–57
Jones MJ, Dunkle LD (1993) Analysis of Cochliobolus carbonum races by PCR amplification with arbitrary and gene-specific primers. Phytopathology 83:366–370
Kema GHJ, Verstappen ECP, Todorova M, Waalwijk C (1996) Successful crosses and molecular tetrad and progeny analyses demonstrate heterothallism in Mycosphaerella graminicola. Curr Genet 30:251–258
Luttrell ES (1973) Loculoascomycetes. In: Ainsworth GC, Sparrow FK, Sussman AS (eds) The fungi, an advanced treatise, vol IVA. Academic Press, New York, pp 135–219
McDonald BA, Pettway RE, Chen RS, Boeger JM, Martinez JP (1995) The population genetics of Septoria tritici (teleomorph Mycosphaerella graminicola). Can J Bot 73: S292–S301
Metzenberg RL, Glass NL (1990) Mating-type and mating strategies in Neurospora. Bioessays 12:53–59
Milgroom MG (1996) Recombination and the multilocus structure of fungal populations. Annu Rev Phytopathol 34:457–477
Perrière G, Gouy M (1996) WWW-Query: an on-line retrieval system for biological sequence banks. Biochimie 78:364–369
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual (2nd edn) Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Sanderson FR (1972) A Mycosphaerella species as the ascogenous state of Septoria tritici Rob ex Desm. N Z J Bot 10:707–709
Sharon A, Yamaguchi K, Christiansen S, Horwitz BA, Yoder OC, Turgeon BG (1996) An asexual fungus has the potential for sexual development. Mol Gen Genet 251:60–68
Shaw MW, Royle DJ (1989) Airborne inoculum as a major source of Septoria tritici (Mycosphaerella graminicola) infections in winter wheat crops in the UK. Plant Pathol 38:35–43
Shearer BL, Skovmand B, Wilcoxson RD (1977) Hordeum jubatum as a source of inoculum of Septoria avenae f. sp. triticea and S. passerinii. Phytopathology 67:1338–1341
Sheldon AL (1969) Equitability indices: dependence on the species count. Ecology 50:466–467
Silva-Hanlin DMW, Hanlin RT (1999) Small subunit ribosomal RNA gene phylogeny of several loculoascomycetes and its taxonomic implications. Mycol Res 103:153–160
Singh G, Ashby AM (1998) Cloning of the mating type loci from Pyrenopeziza brassicae reveals the presence of a novel mating type gene within a discomycete MAT 1-2 locus encoding a putative metallothionen-like protein. Mol Microbiol 30:799–806
Tatusova TA, Madden TL (1999) BLAST 2 Sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett 174:247–250
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Turgeon BG (1998) Application of mating type gene technology to problems in fungal biology. Annu Rev Phytopathol 36:115–137
Turgeon BG, Yoder OC (2000) Proposed nomenclature for mating type genes of filamentous Ascomycetes. Fungal Genet Biol 31: 1–5
Waalwijk C, Mendes O, Verstappen ECP, de Waard MA, Kema GHJ (2002) Isolation and characterization of the mating-type idiomorphs from the wheat septoria leaf blotch fungus Mycosphaerella graminicola. Fungal Genet Biol 35:277–286
Yun S-H, Berbee ML, Yoder OC, Turgeon BG (1999) Evolution of the fungal self-fertile reproductive life style from self-sterile ancestors. Proc Natl Acad Sci USA 96:5592–5597
Yun S-H, Arie T, Kaneko I, Yoder OC, Turgeon BG (2000) Molecular organization of mating type loci in heterothallic, homothallic, and asexual Gibberella / Fusarium species. Fungal Genet Biol 31:7–20
Zhan J, Mundt CC, McDonald BA (1998) Measuring immigration and sexual reproduction in field populations of Mycosphaerella graminicola. Phytopathology 88:1330–1337
Acknowledgements
This work was supported by USDA CRIS project 3602-22000-011-00D. We thank Dave Long (USDA-ARS, Cereal Disease Laboratory) for providing S. passerinii -infected barley leaves from Minnesota and North Dakota. Larry Dunkle and Jin-Rong Xu provided helpful comments on a previous draft of the manuscript. Published as paper 16941, Purdue University Agricultural Experiment Station
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Cerdá-Olmedo
Rights and permissions
About this article
Cite this article
Goodwin, S.B., Waalwijk, C., Kema, G.H.J. et al. Cloning and analysis of the mating-type idiomorphs from the barley pathogen Septoria passerinii . Mol Gen Genomics 269, 1–12 (2003). https://doi.org/10.1007/s00438-002-0795-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-002-0795-x