Abstract
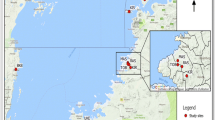
The New World screwworm, Cochliomyia hominivorax, is a major parasite that causes myiasis in livestock, humans, and other warm-blooded animals in the western hemisphere. There is a permanent biological border that is maintained between Panama and Colombia, as it has been eradicated from North and Central America. However, it still exists in much of the Caribbean and South America causing an estimated annual loss of $3.6 billion dollars in South America alone. Less information is available for C. hominivorax in the Caribbean. Thus, here we examined its presence and genetic landscape in order to gain insights into this fly’s distribution in this region. First, through sampling efforts, novel GPS (Global Positioning System) coordinates were collected. Second, the environmental correlates of those presence points were examined. Next, samples were sequenced in order to obtain a pairwise ΦIT genetic distance matrix. And lastly, this matrix was used to create a genetic landscape of divergence. The results of the genetic landscape show flies as more diverse in Trinidad and Tobago and less diverse in the Dominican Republic. This is perhaps due to the proximity of Trinidad to Venezuela and gene flow may be occurring between these two areas. This information will aid in screwworm surveillance and control programs by providing environmental correlates and a view into the distribution of these flies.



Similar content being viewed by others
Data availability
Sequence Read Archive (SRA) accession number: PRJNA775625.
References
Adams T, Reinecke J (1979) The reproductive physiology of the screwworm, Cochliomyia hominivorax (Diptera: Calliphoridae). I Oogenesis J Med Entomol 15:472–483
Altuna M, Hickner PV, Castro G, Mirazo S, Pérez de León AA, Arp AP (2021) New World screwworm (Cochliomyia hominivorax) myiasis in feral swine of Uruguay: One Health and transboundary disease implications. Parasit Vectors 14:1–9
Baumhover AH (2002) A personal account of developing the sterile insect technique to eradicate the screwworm from Curacao, Florida and the southeastern United States. Fla Entomol 85:666–673
Catchen J, Hohenlohe PA, Bassham S, Amores A, Cresko WA (2013) STACKS: an analysis tool set for population genomics. Mol Ecol 22:3124–3140
Coronado A, Kowalski A (2009) Current status of the New World screwworm Cochliomyia hominivorax in Venezuela. Med Vet Entomol 23:106–110
European Space Agency (2017) Climate change initiative land cover. https://www.esa-landcover-cci.org/
Fenton R (1961) The screwworm in the Bahamas. Vet Record 73:75–76
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315
Fresia P, Lyra ML, Coronado A, De Azeredo-Espin AML (2011) Genetic structure and demographic history of New World screwworm across its current geographic range. J Med Entomol 48:280–290
Fresia P, Azeredo-Espin AML, Lyra ML (2013) The phylogeographic history of the New World screwworm fly, inferred by approximate Bayesian computation analysis. PLoS ONE 8:e76168
Fresia P, Silver M, Mastrangelo T, De Azeredo-Espin AML, Lyra ML (2014) Applying spatial analysis of genetic and environmental data to predict connection corridors to the New World screwworm populations in South America. Acta Trop 138:S34–S41
Fresia P, Pimentel S, Iriarte V, Marques L, Durán V, Saravia A, Novas R, Basika T, Ferenczi A, Castells D (2021) Historical perspective and new avenues to control the myiasis-causing Cochliomyia hominivorax fly in Uruguay. Agrociencia Uruguay 25(2):e974
Garcia R, Mendez L, Serrano E, Morales TG, and Vreysen M (2007) Insecticidal wound treatment of livestock on Isla de la Juventud, Cuba: an efficient suppression method of new world screwworm Cochliomyia hominivorax prior to the release of sterile insects. In: Vreysen MJB, Robinson AS, Hendrichs J (eds) Area-wide control of insect pests, 1st edn. Springer, Dordrecht, Netherlands, pp 393–403
Hightower B, Adams A, Alley D (1965) Dispersal of released irradiated laboratory-reared screw-worm flies. J Econ Entomol 58:373–374
Hightower B, Davis R, Baumhover A, Graham O (1966) Seasonal abundance of the screw-worm in northern Mexico. J Econ Entomol 59:416–420
Krafsur E (1987) Climatological correlates of screwworm (Cochliomyia hominivorax) abundance in Texas. USA Med Vet Entomol 1:71–80
Krafsur E, Whitten C, Novy J (1987) Screwworm eradication in North and Central America. Parasitol Today 3:131–137
Li H, Durbin R (2010) Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinform 26:589–595
Lindquist AW, Barrett Jr W (1945) Overwintering of Cochliomyia americana at Uvalde, Texas. J Econ Entomol 38:77–83
Lyra M, Klaczko L, Azeredo-Espin A (2009) Complex patterns of genetic variability in populations of the New World screwworm fly revealed by mitochondrial DNA markers. Med Vet Entomol 23:32–42
Mackley JW, Brown HE (1984) Swormlure-4: a new formulation of the swormlure-2 mixture as an attractant for adult screwworms, Cochliomyia hominivorax (Diptera: Calliphoridae). J Econ Entomol 77:1264–1268
Mangan RL, Thomas DB (1989) Habitat preferences and dispersal patterns in native female screwworm flies (Diptera: Calliphoridae). Ann Entomol Soc Am 82:332–339
Mayer DG, Atzeni MG (1993) Estimation of dispersal distances for Cochliomyia hominivorax (Diptera: Calliphoridae). Environ Entomol 22:368–374
Mulieri PR, Patitucci LD (2019) Using ecological niche models to describe the geographical distribution of the myiasis-causing Cochliomyia hominivorax (Diptera: Calliphoridae) in southern South America. Parasitol Res 118:1077–1086
Parman D (1945) Effect of weather on Cochliomyia americana and a review of methods and economic applications of the study. J Econ Entomol 38:66–76
Phillips PL, Welch JB, Kramer M (2004) Seasonal and spatial distributions of adult screwworms (Diptera: Calliphoridae) in the Panama Canal area, Republic of Panama. J Med Entomol 41:121–129
Rawlins SC (1985) Current trends is screwworm myiasis in the Caribbean region. Vet Parasitol 18:241–250
Rawlins SC, Sang JC (1984) Screwworm myiasis in Jamaica and proposals for its eradication. Int J Pest Manag 30:125–129
Rodríguez-Hidalgo R, Tapia-Chiriboga A, Arciniegas S, Vanwambeke SO, Benítez-Ortiz W (2019) Epidemiological analysis of the New World screwworm (Cochliomyia hominivorax) in Ecuador. Transbound Emerg Dis 66:968–977
Scott MJ, Benoit JB, Davis RJ, Bailey ST, Varga V, Martinson EO, Hickner PV, Syed Z, Cardoso GA, Torres TT (2020) Genomic analyses of a livestock pest, the New World screwworm, find potential targets for genetic control programs. Comm Biol 3:1–14
Snow J, Hofmann H, Baumhover A (1977) The screwworm as a pest on the island of Jamaica and the feasibility of eradication by the sterile insect method. Southwest Entomol 2(4):202–206
Tietjen M, Pérez de León AA, Sagel A, Skoda SR, Phillips PL, Mitchell RD III, Caruth J, Durán U, Musai L, Tortosa S (2022) Geographic population genetic structure of the New World screwworm, Cochliomyia hominivorax (Diptera: Calliphoridae), using SNPs. J Med Entomol 59:874–882
Toledo-Perdomo CE, Skoda SR (2020) Estudio molecular de seis cepas de Cochliomyia hominivorax (Coquerel)(Diptera: Calliphoridae) y Cochliomyia macellaria. Agronomía Mesoamericana 31:433–444
Torres T, Azeredo-Espin A (2009) Population genetics of New World screwworm from the Caribbean: insights from microsatellite data. Med Vet Entomol 23:23–31
Vandergast AG, Perry WM, Lugo RV, Hathaway SA (2011) Genetic landscapes GIS Toolbox: tools to map patterns of genetic divergence and diversity. Molec Ecol Res 11:158–161
Vargas-Terán M, Spradbery J, Hofmann H, Tweddle N (2021) Impact of screwworm eradication programmes using the sterile insect technique. In: Dyck VA, Hendrichs J, Robinson AS (eds) Sterile insect technique: principles and practice in area-wide integrated pest management, 2nd edn. CRC Press, Boca Raton, Florida, pp 949–978
Williams D, Gartman S, Hourrigan L (1977) Screwworm eradication in Puerto Rico and the Virgin Islands. World Animal Review 21:31–35
Acknowledgements
The authors would like to thank Steve Skoda (retired ARS) for providing Jamaica GPS coordinates. Pamela Phillips and John Welch (APHIS), Kim Lohmeyer, Alex Arp and Paul Hickner (ARS), and Maxwell Scott (North Carolina State University) for feedback on study. Adalberto Pérez de León (ARS), Robert D. Mitchell (EPA), Joanne Caruth (DFPFF, Trinidad and Tobago), Uziel Durán and Silvia Tortosa (DIEGA, Dominican Republic), and Lisa Musai (MALF, Trinidad and Tobago) for help in obtaining samples used in study.
Funding
This research was supported in part by the U.S. Department of Agriculture, Agricultural Research Service. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Contributions
Mackenzie Tietjen: Conceptualization, Data curation, Methodology, Investigation, Writing—original draft. Vera Pfeiffer: Data curation, Software, Writing—review & editing. Karen Poh: Software, Validation, Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Van Lun Low
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article reports the results of research only. Mention of a proprietary product does not constitute an endorsement or a recommendation by the USDA for its use. The USDA is an equal opportunity provider and employer.
Rights and permissions
About this article
Cite this article
Tietjen, M., Pfeiffer, V. & Poh, K.C. Insights into the genetic landscape and presence of Cochliomyia hominivorax in the Caribbean. Parasitol Res 122, 547–556 (2023). https://doi.org/10.1007/s00436-022-07757-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07757-4