Abstract
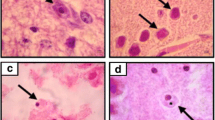
This study was developed in order to describe the early morphological events observed during the invasion of two pathogenic strains of Acanthamoeba (genotype T4); A. castellanii and A. culbertsoni, at the olfactory meatus and cerebral, pulmonary, renal, hepatic and splenic tissues levels, an in vivo invasion study. Histological and immunohistochemical description of the events at 24, 48, 72, and 96 h postintranasal inoculations of BALB/c mice was performed. A. castellanii showed a higher invasion rate than A. culbertsoni, which was only able to reach lung and brain tissue in the in vivo model. The current study supports previous evidence of lack of inflammatory response during the early stages of infection. Acanthamoeba invasion of the CNS and other organs is a slow and contact-dependent process. The early morphological events during the invasion of amoebae include the penetration of trophozoites into different epithelia: olfactory, respiratory, alveolar space, and renal tubule, which resemble the process of amoebae invasion described in corneal tissue. The data suggest that after reaching the nasal epithelium, trophozoites continued invasion, separating and lifting the most superficial cells, then migrating and penetrating between the cell junctions without causing a cytolytic effect on adjacent cells. These results reaffirm the idea that contact-dependent mechanisms are relevant for amoebae of Acanthamoeba genus regardless of the invasion site.



Similar content being viewed by others
References
Abd H, Saeed A, Jalal S, Bekassy AN, Sandström G (2009) Ante mortem diagnosis of amoebic encephalitis in a haematopoietic stem cell transplanted patient. Scand J Infect Dis 41(8):619–622. doi:10.1007/s00436-015-4871-7
Alves DS, Moraes AS, Alves LM, Gurgel-Gonçalves R, Lino Junior RS, Cuba-Cuba CA, Vinaud MC (2016) Experimental infection of T4 Acanthamoeba genotype determines the pathogenic potential. Parasitol Res 115(9):3435–3440. doi:10.1007/s00436-016-5105-3
Arnalich-Montiel F, Martín-Navarro CM, Alió JL, López-Vélez R, Martínez-Carretero E, Valladares B, Piñero JE, Lorenzo-Morales J (2012) Successful monitoring and treatment of intraocular dissemination of Acanthamoeba. Arch Ophthalmol 130:1474–1475
Baig AM (2015) Pathogenesis of amoebic encephalitis: are the amoebae being credited to an 'inside job' done by the host immune response? Acta Trop 148:72–76. doi:10.1016/j.actatropica.2015.04.022
Blanco-Vidal MJ, Catalán-Uribarrena G, López JI, Del Águila C, Magnet A, Pomposo-Gaztelu I, Montejo M (2013) Hemiparesia izquierda en un paciente diabético: encefalitis granulomatosa crónica por Acanthamoeba. Rev Neurol 56:187–188
Booton GC, Kelly DJ, Chu YW, Seal DV, Houang E, Lam DS, Byers TJ, Fuerst PA (2002) 18S ribosomal DNA typing and tracking of Acanthamoeba species isolates from corneal scrape specimens, contact lenses, lens cases, and home water supplies of Acanthamoeba keratitis patients in Hong Kong. J Clin Microbiol 40:1621–1625
Chávez-Munguía B, Salazar-Villatoro L, Omaña-Molina M, Espinosa-Cantellano M, Ramírez-Flores E, Lorenzo-Morales J, Martínez-Palomo A (2016) Acanthamoeba culbertsoni: electron-dense granules in a highly virulent clinical isolate. J Eukaryot Microbiol. doi:10.1111/jeu.12321
Culbertson CG, Smith JW, Cohen HK, Minner JR (1959) Experimental infection of mice and monkey by Acanthamoeba. Am J Pathol 35:185–197
Culbertson CG, Ensminger PW, Overton WM (1966) Hartmannella (Acanthamoeba). Experimental chronic, granulomatous brain infections produced by new isolates of low virulence. Am J Clin Pathol 46(3):305–314
Das S, Saha R, Rani M, Goyal R, Shah D, Asish JK (2016) Central nervous system infection due to Acanthamoeba: a case series. Trop Parasitol 6(1):88–91. doi:10.4103/2229-5070.175130
Doan N, Rozansky G, Nguyen HS, Gelsomino M, Shabani S, Mueller W, Johnson V (2015) Granulomatous amebic encephalitis following hematopoietic stem cell transplantation. Surg Neurol Int 6:S459–S462. doi:10.4103/2152-7806.166788
Fung KT, Dhillon AP, McLaughlin JE, Lucas SB, Davidson B, Rolles K, Patch D, Burroughs AK (2008) Cure of Acanthamoeba cerebral abscess in a liver transplant patient. Liver Transpl 14:308–312
Górnik K, Kuźna-Grygiel W (2005) Histological studies of selected organs of mice experimentally infected with Acanthamoeba spp. Folia Morphol (Warsz) 64(3):161–167
Grunnet ML, Cannon GH, Kushner JP (1981) Fulminant amebic meningoencephalitis due to Acanthamoeba. Neurology 31(2):174–176
Kasprzak W, Mazur T, Rucka A (1974) Studies on some pathogenic strains of free-living amoebae isolated from lakes in Poland. Ann Soc Belg Med Trop 54(4–5):351–357
Kaushal V, Chhina DK, Kumar R, Pannu HS, Dhooria HP, Chhina RS (2008) Acanthamoeba encephalitis. J Med Microbiol 26:182–184
Khan NA, Siddiqui R (2009) Acanthamoeba affects the integrity of human brain microvascular endothelial cells and degrades the tight junction proteins. Int J Parsitol 39:1611–1616
Kinde H, Read DH, Daft BM, Manzer M, Nordhausen RW, Kelly DJ, Fuerst PA, Booton G, Visvesvara GS (2007) Infections caused by pathogenic free-living amebas (Balamuthia mandrillaris and Acanthamoeba sp.) in horses. J Vet Diagn Investig 19(3):317–322
Koide J, Okusawa E, Ito T, Mori S, Takeuchi T, Itoyama S, Abe T (1998) Granulomatous amebic encephalitis caused by Acanthamoeba in a patient with systemic lupus erythematosus. Clin Rheumatol 17(4):329–332
Lorenzo-Morales J, Ortega-Rivas A, Martínez E, Khoubbane M, Artigas P, Periago MV, Foronda P, Abreu-Acosta N, Valladares B, Mas-Coma S (2006) Acanthamoeba isolates belonging to T1, T2, T3, T4 and T7 genotypes from environmental freshwater samples in the Nile delta region. Acta Trop 100:63–69
Ma P, Visvesvara GS, Martinez AJ, Theodore FH, Daggett PM, Sawyer TK (1990) Naegleria and Acanthamoeba infections: review. Rev Infect Dis 12(3):490–513
Marciano-Cabral F, Cabral G (2003) Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev 2:273–307
Martinez AJ (1982) Acanthamoebiasis and immunosuppression. Case report. J Neuropathol Exp Neurol 41(5):548–557
Martinez JA (1991) Infection of the central nervous system due to Acanthamoeba. Rev Infect Dis 13(Suppl 5):S399–S402
Martinez AJ, Janitschke K (1985) Acanthamoeba, an opportunistic microorganism: a review. Infection 13(6):251–256
Martinez AJ, Visvesvara GS (1997) Free-living, amphizoic and opportunistic amebas. Brain Pathol 7(1):583–598
Martinez JA, Markowitz SM, Duma JR (1975) Experimental pneumonitis and encephalitis caused by Acanthamoeba in mice: pathogenesis and ultrastructural features. J Infect Dis 131:692–699
Massilamany C, Marciano-Cabral F, Rocha-Azevedo BD, Jamerson M, Gangaplara A, Steffen D, Zabad R, Illes Z, Sobel RA, Reddy J (2014) SJL mice infected with Acanthamoeba castellanii develop central nervous system autoimmunity through the generation of cross-reactive T cells for myelin antigens. PLoS One 9(5):e98506. doi:10.1371/journal.pone.0098506
Memari F, Niyyati M, Haghighi A, Seyyed Tabaei SJ, Lasjerdi Z (2015) Ocurrence of pathogenic Acanthamoeba genotypes in nasal swabs of cancer patients in Iran. Parasitol Res 114(5):197–212
Omaña-Molina M, González-Robles A, Salazar-Villatoro L, Lorenzo-Morales J, Cristóbal-Ramos AR, Hernández-Ramírez VI, Talamás-Rohana P, Méndez-Cruz AR, Martínez-Palomo A (2013) Reevaluating the role of Acanthamoeba proteases in tissue invasion: observation of cytopathogenic mechanisms on MDCK cell monolayers and hamster corneal cells. Biomed Res Int. doi:10.1155/2013/461329
Omaña-Molina M, Vanzzini-Zago V, Hernandez-Martinez D, Gonzalez-Robles A, Salazar-Villatoro L, Ramirez-Flores E, Oregon-Miranda E, Lorenzo-Morales J, Martinez-Palomo A (2016) Acanthamoeba genotypes T3 and T4 as causative agents of amoebic keratitis in Mexico. Parasitol Res 115(2):873–878. doi:10.1007/s00436-015-4821-4
Page FC (1988) A new key to freshwater and soil Gymnamoebae with instructions for culture. Culture Collections of Algae and Protozoa. Freshwater Biological Association. Ambleside, Cumbria, England, pp 92–96
Petry F, Torzewski M, Bohl J, Wilhelm-Schwenkmezger T, Scheid P, Walochnik J, Zöller L, Bhakdi S, Lackner KJ (2006) Early diagnosis of Acanthamoeba infection during routine cytological examination of cerebrospinal fluid. J Clin Microbiol 44:1903–1904. doi:10.1128/JCM.44.5.1903-1904.2006
Rojas-Hernández S, Jarillo-Luna A, Rodríguez-Monroy M, Moreno-Fierros L, Campos-Rodríguez R (2004) Immunohistochemical characterization of the initial stages of Naegleria fowleri meningoencephalitis in mice. Parasitol Res 94(1):31–36
Salameh A, Bello N, Becker J, Zangeneh T (2015) Fatal granulomatous amoebic encephalitis caused by Acanthamoeba in a patient with kidney transplant: a case report. Open Forum Infect Dis 2(3). doi:10.1093/ofid/ofv104
Satlin MJ, Graham JK, Visvesvara GS, Mena H, Marks KM, Saal SD, Soave R (2013) Fulminant and fatal encephalitis caused by Acanthamoeba in a kidney transplant recipient: case report and literature review. Transpl Infect Dis 15:619–626
Shukla D, Rumpa S, Mayuri R, Ritika G, Dheeraj S, Jhajjar KA (2016) Central nervous system infection due to Acanthamoeba: a case series. Trop Parasitol 6(1):88–91
Siddiqui R, Emes R, Elsheikha H, Khan NA (2011) Area 51: how do Acanthamoeba invade the central nervous system? Trends Parasitol 27(5):185–189
Singhal T, Bajpai A, Kalra V, Kabra SK, Samantaray JC, Satpathy G, Gupta AK (2001) Successful treatment of Acanthamoeba meningitis with combination oral antimicrobials. Pediatr Infect Dis J 20(6):623–627
Thamtam VK, Uppin MS, Pyal A, Kaul S, Rani JY, Sundaram C (2016) Fatal granulomatous amoebic encephalitis caused by Acanthamoeba in a newly diagnosed patient with systemic lupus erythematosus. Neurol India 64:101–104
Visvesvara GS (2013) Infections with free-living amebae. Handb Clin Neurol 114:153–168
Visvesvara GS, Moura H, Schuster FL (2007) Pathogenic and opportunistic free-livingamoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri and Sappinia diploidea. Immunol Med Microbiol 50(1):1–26
Walochnik J, Haller-Schober EM, Kölli H, Picher O, Obwaller A, Aspöck H (2000) Discrimination between clinically relevant and nonrelevant Acanthamoeba strains isolated from contact lens-wearing keratitis patients in Austria. J Clin Microbiol 38(11):3932–3936
Young AL, LeBoeuf NR, Tsiouris SJ, Husain S, Grossman ME (2010) Fatal disseminated Acanthamoeba infection in a liver transplant recipient immunocompromised by combination therapies for graft-versus-host disease. Transpl Infect Dis 12:529–537
Acknowledgements
This work was funded by the UNAM-FESI grant no. FESI-DIP-PAPCA-2014-13. We deeply appreciate the valuable support of Lizbeth Salazar Villatoro for her technical assistance in amoebae cultures, Miriam Romero Grijalva for her assistance in handling mice, and Carmen Guadalupe Mondragon Huerta and Leticia Verdín Terán for their immunohistochemistry assistance. Finally we thank Maria Rosa Avila responsible for the Neuromorphology Laboratory, of the School of Superior Studies Iztacala, National Autonomous University of Mexico, for providing the equipment to carry out the histological processes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Omaña-Molina, M., Hernandez-Martinez, D., Sanchez-Rocha, R. et al. In vivo CNS infection model of Acanthamoeba genotype T4: the early stages of infection lack presence of host inflammatory response and are a slow and contact-dependent process. Parasitol Res 116, 725–733 (2017). https://doi.org/10.1007/s00436-016-5338-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5338-1