Abstract
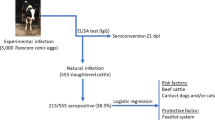
Human toxocariasis may be acquired by eating raw chicken liver. However, there are no reports on the prevalence of natural infection of chickens with Toxocara. The aim of this study was to evaluate the presence of anti-Toxocara antibodies as indicators of natural infection with Toxocara, in free-range chickens from Espírito Santo State, Brazil. An ELISA test with secretory and excretory Toxocara canis antigens was used. Negative controls were 20 industrial chickens reared in a high hygiene standard environment. Positive control serum was from a chicken infected with embryonated eggs of T. canis. Sera were adsorbed with Ascaridia galli extract to reduce cross-reactivity. Cut-off was the mean plus four times the standard deviation of optical density (OD) in negative group. One hundred and fifty-seven sera from free-range chicken were investigated. Results showed 58.5 % of the chickens were positive with ELISA test; 12.7 % had OD over the positive control and may be considered as true infected chickens. The results between the cut-off and the positive control may include infections with low titers of antibodies or may represent serum scar of past infection or may be the result of cross-reaction with other nematodes rather than A. galli which is used for the adsorption of sera. In conclusion, high prevalence of Toxocara sp. antibodies demonstrates natural infection of free-range chickens from Espírito Santo State which may represent a risk of infection with this nematode in people who have the habit of eating raw or undercooked chicken meat or viscera. The results also suggest that chickens may be useful as sentinels to detect soil contaminated with Toxocara eggs.

Similar content being viewed by others
References
Agnihotri RK, Bhatia BB, Kumar D (1987) Visceral larva migrans: 1 migratory behavior of Toxocara canis larvae in golden hamster and chicken. Indian J Anim Sci 57:853–855
Azizi S, Oryan A, Sadjjadi SM, Zibaei M (2007) Histopathologic changes and larval recovery of Toxocara cati in experimentally infected chickens. Parasitol Res 102:47–52
Beaver PC (1956) Parasitological reviews: larva migrans. Exp Parasitol 5:587–621
Bowman DB (2003) Helminths. In: Bowman DB (ed) Georgis’ parasitology for veterinarians, 8th edn. WB Saunders Co, Philadelphia Pa, pp 115–243
Cancrini G, Bartoloni A, Zaffaroni E, Guglielmetti P, Gamboa H, Nicoletti A, Genchi C (1998) Seroprevalence of Toxocara canis-IgG antibodies in two rural Bolivian communities. Parassitologia 4:473–475
de Savigny DH, Voller A, Woodruff AW (1979) Toxocariasis: serological diagnosis by enzyme immunoassay. J Clin Pathol 32:284–288
Despommier D (2003) Toxocariasis: clinical aspects epidemiology medical ecology and molecular aspects. Clin Microbiol Rev 16:265–272
Dutra GF, Pinto NS, da Costa de Avila LF, de Lima TP, da Hora VP, Martins LH, Berne ME, Scaini CJ (2013) Evaluation of the initial and chronic phases of toxocariasis after consumption of liver treated by freezing or cooling. Parasitol Res 112:2171–2175
Elefant GR, Shimizu SH, Sanchez MC, Jacob CM, Ferreira AW (2006) A serological follow-up of toxocariasis patients after chemotherapy based on the detection of IgG IgA and IgE antibodies by enzyme-linked immunosorbent assay. Journal of Clinical Laboraopry Analysis 20:164–172
Fragoso RP, Monteiro MBM, Lemos EM, Pereira FEL (2011) Anti-Toxocara antibodies detected in children attending elementary school in Vitória State of Espírito Santo Brazil: prevalence and associated factors. Rev Soc Brasil Med Trop 44:461–466
Galvin TJ (1964) Experimental Toxocara canis infections in chickens and pigeons. J Parasitol 50:124–127
Gargili A, Tuzer E, Gulanber A, Toparlak M, Efil I, Keles V, Ulutas M (1999) Experimental visceral larva migrans in chicken with Toxocara canis. Turk J Vete Anim Sci 23:431–434
Hoffmeister B, Glaeser S, Flick H, Pornschlegel S, Suttorp N, Bergmann F (2007) Cerebral toxocariasis after consumption of raw duck liver. Am J Trop Med Hyg 76:600–602
Jacquier P, Gottstein B, Stringelin Y, Eckert J (1991) Immunodiagnosis of toxocarosis in humans: evaluation of a new enzyme-linked immunosorbent assay kit. J Clin Microbiol 29:1831–1835
Kanamura HY, Hoshino-Shimizu S, Silva LC (1981) Solubilization of antigen S mansoni adult worms for the passive hemagglutination test. Rev Inst Med Trop Sao Paulo 23:92–95
Lee KT, Min HK, Chung PR, Chang JK (1976) Studies on the inducing possibility of human visceral larva migrans associated with eating habit of raw liver of domestic animals. Korean J Parasitol 14:51–60
Lynch NR, Wilkes LK, Hodgen AN, Turner KJ (1988) Specificity of Toxocara ELISA in tropical populations. Parasite Immunol 10:323–337
Maruyama S, Nino T, Yamamoto K, Katsube Y (1994) Parasitism of Toxocara canis larvae in chickens inoculated with the Ascarid eggs. J Vet Med Sci 56:139–141
Morimatsu Y, Akao N, Akiyoshi H, Kawazu T, Okabe Y, Aizawa H (2006) A familial case of visceral larva migrans after ingestion of raw chicken livers: appearance of specific antibody in bronchoalveolar lavage fluid of the patients. Am J Trop Med Hyg 75:303–306
Nagakura K, Tachibana H, Kaneda Y, Kato Y (1989) Toxocariasis possibly caused by ingesting raw chicken. J Infect Dis 160:735–736
Noh Y, Hong ST, Yun JY, Park HK, Oh JH, Kim YE, Jeon BS (2012) Meningitis by Toxocara canis after ingestion of raw ostrich liver. J Korean Med Sci 27:1105–1108
Okoshi S, Usui M (1968) Experimental studies on Toxocara leonina: VI experimental infection of mice chickens and earthworms with Toxocara leonina, Toxocara canis and Toxocara cati. Nihon Juigaku Zasshi (Jap J Vet Sci) 30:151–166
Oryan A, Sadjjadi SM, Azizi S (2010) Longevity of Toxocara cati larvae and pathology in tissues of experimentally infected chickens. Korean J Parasitol 48:79–80
Pawlowski Z (2001) Toxocariasis in humans: clinical expression and treatment dilemma. J Helminthol 75:299–305
Romasanta A, Romero JL, Arias M, Sánchez-Andrade R, López C, Suárez JL, Diaz P, Diez-Banos P, Morrondo P, Paz-Silva A (2003) Diagnosis of parasitic zoonoses by immunoenzymatic assays-analysis of crossreactivity among the excretory/secretory antigens of Fasciola hepatica Toxocara canis and Ascaris suum. Immunol Invest 32:131–142
Rubinsky-Elefant G, Jacob CM, Kanashiro EHY, Peres BA (2001) Toxocaríase. pp323-332 in Ferreira AW & Ávila SLM(Eds) Diagnóstico laboratorial das principais doenças infecciosas e auto-imunes Rio de Janeiro Editora Guanabara Koogan
Rubinsky-Elefant G, da Silva-Nunes M, Malafronte RS, Muniz PT, Ferreira MU (2008) Human toxocariasis in rural Brazilian Amazonia: seroprevalence risk factors and spatial distribution. Am J Trop Med Hyg 79:93–98
Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU (2010) Human toxocariasis: diagnosis worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol 104:3–23
Salem G, Schantz P (1992) Toxocaral visceral larva migrans after ingestion of raw lamb liver. Clin Infect Dis 15:743–744
Sprent JF (1956) The life history and development of Toxocara cati (Schrank 1788) in the domestic cat. Parasitology 46:54–78
Stürchler D, Weiss N, Gassner M (1990) Transmission of toxocariasis. J Infect Dis 162:571
Taira K, Permin A, Kapel CM (2003) Establishment and migration pattern of Toxocara canis larvae in chickens. Parasitol Res 90:521–123
Taira K, Saeed I, Permin A, Kapel CM (2004) Zoonotic risk of Toxocara canis infection through consumption of pig or poultry viscera. Vet Parasitol 121:115–124
Taira K, Saitoh Y, Kapel CM (2011) Toxocara cati larvae persist and retain high infectivity in muscles of experimentally infected chickens. Vet Parasitol 180:287–291
Taira K, Saitoh Y, Okada N, Sugiyama H, Kapel CM (2012) Tolerance to low temperatures of Toxocara cati larvae in chicken muscle tissue. Vet Parasiol 189:383–386
Tuzer E, Toparlak M, Gargili A, Gulanber A, Keles V, EfilI-Esatgil MU (2002) Visceral larva migrans in mice caused by eating Toxocara canis infected chicken livers. Turk J Vet Anim Sci 26:293–297
Yoshikawa M, Nishiofuku M, Moriya K, Ouji Y, Ishizaka S, Kasahara K, Mikasa K, Hirai T, Mizuno Y, Ogawa S, Nakamura T, Maruyama H, Akao N (2008) A familial case of visceral toxocariasis due to consumption of raw bovine liver. Parasitolol Int 57:525–529
Acknowledgements
We thank to Dr. Guita Rubinski-Elefant providing the TES antigen of Toxocara canis.
Conflict of interest
The authors declare that they have no competing of interest.
Ethical statement
The authors assert that all procedures contributing to this work comply with the ethical standards of the national and institutional guides on the care and use of laboratory animals. The research was approved by the Ethics, Bioethics, and Animal Welfare Committee of Vila Velha University.
Financial support
This research was supported by the Fundação Nacional de Desenvolvimento do Ensino Superior Particular (FUNADESP) and by the intramural funding of University Vela Velha.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Campos-da-Silva, D.R., da Paz, J.S., Fortunato, V.R. et al. Natural infection of free-range chickens with the ascarid nematode Toxocara sp.. Parasitol Res 114, 4289–4293 (2015). https://doi.org/10.1007/s00436-015-4669-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4669-7