Abstract
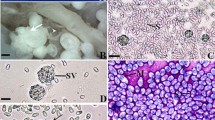
A previously unrecognised fish-infecting microsporidia (Loma psittaca n. sp.), found adherent to the intestinal mucosa of the freshwater puffer fish Colomesus psittacus (Teleostei, Tetraodontidae) from lower Amazon River, was described based on light and transmission electron microscope and phylogenetic analysis. The whitish xenoma was completely filled by numerous spores, including several developmental stages of the parasite. In all of these stages, the nuclei were monokaryotic. The merogonial plasmodium divided by binary fission and the sporont gave rise to disporoblastic ovoid spores measuring 4.2 ± 0.4 × 2.8 ± 0.4 μm. In mature spores, the polar filament was arranged in 10–11 (rarely 12) coils in one row in turn of posterior vacuole. The polaroplast had two distinct regions around the manubrium. The polyribosomes were organised in coiled tapes. The small subunit rRNA gene was sequenced and maximum parsimony analysis placed the microsporidian described here in the clade that includes the genera Ichthyosporidium, Loma and Pseudoloma. Based on differences from previously described microsporidians, such as ultrastructural characteristics of the xenoma, developmental stages including the spore and phylogenetic analysis supported the recognition of a new species, herein named L. psittaca n. sp.



Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local aligment search tool. J Parasitol 215:403–410
Azevedo C, Matos E (2002) Fine structure of a new species, Loma myrophis (phylum Microsporidia), parasite of the Amazonian fish Myrophis platyrhynchus (Teleostei, Ophichthidae). Eur J Protistol 37:445–452
Azevedo C, Matos E (2003) Amazonspora hassar n. gen. and n. sp. (phylum Microsporidia, fam Glugeidae), a parasite of the Amazonian teleost Hassar orestis (fam. Doradidae). J Parasitol 89:336–341
Baquero E, Rubio M, Moura INS, Pieniazek J, Jordana R (2005) Myosporidium merluccius n. g., n. sp. infecting muscle of commercial hake (Merluccius sp.) from fisheries near Namibia. J Eukaryot Microbiol 52:476–483
Bekhti M, Bouix G (1985) Loma salmonae (Putz, Hoffmann et Dunbar, 1965) et Loma diplodae n. sp., microsporidies parasites de branchies de poissons téléostéens: implantation et données ultrastructurales. Protistologica 21:47–59
Canning EU, Lom J, Nicholas JP (1982) Genus Glugea Thélohan, 1891 (Phylum Microspora): redescription of the type species Glugea anomala (Moniez, 1887) and recognition of its sporogonic development within sporophorous vesicles (pansporoblastic membranes). Protistologica 18:193–210
Casal G, Matos E, Teles-Grilo ML, Azevedo C (2008) A new microsporidian parasite, Potaspora morhaphis n. gen., n. sp. (Microsporidia) infecting the Teleostean fish, Potamorhaphis guianensis from the River Amazon. Morphological, ultrastructural and molecular characterization. Parasitology 135:1053–1064
Cheney SA, Lafranchi-Tristem NJ, Canning EU (2000) Phylogenetic relationships of Pleistophora-like Microsporidia based on small subunit ribosomal DNA sequences and implications for the source of Trachipleistophora hominis infections. J Eukaryot Microbiol 47:280–287
Docker MF, Devlin RH, Richard J, Kent ML (1997) Sensitive and specific polymerase chain reaction assay for detection of Loma salmonae (Microsporea). Dis Aquat Org 29:41–48
Faye N, Toguebaye BS, Bouix G (1995) On the cytology and development of Loma boopsi n. sp. (Microspora, Glugeidae), parasite of Boops boops (Pisces, Teleostei, Sparidae) from the coasts of Senegal. Arch Protistenkd 146:85–93
Fomena A, Coste F, Bouix G (1992) Loma camerounensis sp. nov. (Protozoa: Microsporida) a parasite of Oreochromis niloticus Linnaeus, 1757 (Teleost: Cichlidae) in fish-rearing ponds in Melen, Yaoundé, Cameroon. Parasitol Res 78:201–208
Hillis DM, Dixon MT (1991) Ribosomal DNA: molecular evolution and phylogenetic inference. Q Rev Biol 66:411–453
Larsson JIR (1999) Identification of Microsporidia. Acta Protozool 38:161–197
Lom J (2002) A catalogue of described genera and species of microsporidians parasitic in fish. Syst Parasitol 53:81–99
Lom J, Dyková I (1992) Protozoan parasites of fishes. Elsevier, Amsterdam, p 315
Lom J, Nilsen F (2003) Fish microsporidia: fine structural diversity and phylogeny. Int J Parasitol 33:107–127
Lom J, Pekkarinen M (1999) Ultrastructural observations on Loma acerinae (Jírovec, 1930) comb. nov. (phylum Microsporidia). Acta Protozool 38:61–74
Loubès C, Maurand J, Gasc C, Buron I, Barral J (1984) Étude ultrastructurale de Loma dimorpha n. sp., microsporidie parasite de poissons Gobiidae languedociens. Protistologica 20:579–589
Matos E, Azevedo C (2004) Ultrastructural description of Microsporidium brevirostris sp. n., parasite of the teleostean Brachyhypopomus brevirostris (Hypopomidae) from the Amazon River. Acta Protozool 43:261–267
Matos E, Corral L, Azevedo C (2003) Ultrastructural details of the xenoma of Loma myrophis (phylum Microsporidia) and extrusion of the polar tube during autoinfection. Dis Aquat Org 54:203–207
Matthews JL, Brown AMV, Larison K, Bishop-Stewart JK, Rogers P, Kent ML (2001) Pseudoloma neurophilia n. g., n. sp., a new microsporidium from the central nervous system of the zebrafish (Danio rerio). J Eukaryot Microbiol 48:227–233
Morrison CM, Sprague V (1981) Electron microscopical study of a new genus and new species of microsporida in the gills of Atlantic cod Gadus morhua L. J Fish Dis 4:15–32
Morrison CM, Sprague V (1983) Loma salmonae (Putz, Hoffman and Dunbar, 1965) in the rainbow trout, Salmo gairdneri Richarson, and L. fontinalis sp. nov. (Microsporida) in the brook trout, Salvelinus fontinalis (Mitchill). J Fish Dis 6:345–353
Nilsen F (2000) Small subunit ribosomal DNA phylogeny of microsporidia with particular reference to genera that infect fish. J Parasitol 86:128–133
Ogawa K, Yokoyama H (1998) Parasitic diseases of cultured marine fish in Japan. Fish Pathol 33:303–309
Putz RE, Hoffman GL, Dunbar CE (1965) Two new species of Pleistophora (Microsporidea) from North America fish with a synopsis of Microsporidea of freshwater and euryhaline fishes. J Protozool 12:228–236
Sandeep BV, Kalavati C (1985) A new microsporidian, Loma trichiuri n. sp., from the gill of a marine fish, Trichiurus salva Cuv. (Trichiuridae). Indian J Parasitol 9:257–259
Shaw RW, Kent ML, Docker MF, Brown AMV, Devlin RH, Adamson ML (1997) A new species of Loma (Microsporea) in shiner perch (Cymatogaster aggregata). J Parasitol 83:296–301
Sprague V, Becnel JJ, Hazard EI (1992) Taxonomy of phylum Microspora. Crit Rev Microbiol 18:285–395
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thompson JD, Higgins DG, Gilson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Vossbrinck CR, Baker MD, Didier ES, Debrunner-Vossbrinck BA, Shadduck JA (1993) Ribosomal DNA sequences of Encephalitozoon hellem and Encephalitozoon cuniculi: species identification and phylogenetic construction. J Eukaryot Microbiol 40:354–362
Acknowledgements
This work partially supported by the Eng. A. Almeida Foundation (Porto, Portugal), PhD grant from “CESPU” (G. Casal), “CNPq” and “CAPES”–Brazil. We would like to thank the iconographic work of Joana Carvalheiro and João Carvalheiro. We assure that this work complies with the current laws of our countries where this was performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Casal, G., Matos, E., Teles-Grilo, M.L. et al. Morphological and genetical description of Loma psittaca sp. n. isolated from the Amazonian fish species Colomesus psittacus . Parasitol Res 105, 1261–1271 (2009). https://doi.org/10.1007/s00436-009-1547-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1547-1