Abstract
Purpose
The preoperative diagnosis of endometriosis associated ovarian cancer (EAOC) remains challenging for lack of effective diagnostic biomarker. We aimed to study clinical characteristics and develop a nomogram for diagnosing EAOC before surgery.
Methods
A total of 87 patients with EAOC and 348 patients with ovarian endometrioma (OEM) were enrolled in our study. Least absolute shrinkage and selection operator (LASSO) regression and Logistic regression were utilized to select variables and construct the prediction model. The performance of the model was assessed using receiver operating characteristic (ROC) analyses and calibration plots, while decision curve analyses (DCAs) were conducted to assess clinical value. Bootstrap resampling was used to evaluated the stability of the model in the derivation set.
Results
The EAOC patients were older compared to the OEM patients (46.41 ± 9.62 vs. 36.49 ± 8.09 year, P < 0.001) and proportion of postmenopausal women was higher in EAOC group than in the OEM group (34.5 vs. 1.5%, P < 0.001). Our prediction model, which included age at diagnosis, tumor size, cancer antigen (CA) 19–9 and risk of ovarian malignancy algorithm (ROMA), demonstrated an area under the curve (AUC) of 0.858 (95% confidence interval (CI): 0.795–0.920) in the derivation set (N = 304) and an AUC of 0.870 (95% CI: 0.779–0.961) in the validation set (N = 131). The model fitted both the derivation (Hosmer–Lemeshow test (HL) chi-square = 12.600, P = 0.247) and the validation (HL chi-square = 8.210, P = 0.608) sets well.
Conclusion
Compared to patients with OEM, those with EAOC exhibited distinct clinical characteristics. Our four-variable prediction model demonstrated excellent performance in both the derivation and validation sets, suggesting its potential to assist with preoperative diagnosis of EAOC.
Similar content being viewed by others
Introduction
Endometriosis (EM) is one of the most common gynecological diseases, and affects approximately 190 million women worldwide (Zondervan et al. 2020). Ovarian endometrioma (OEM) is one of the subtypes of EM along with superficial peritoneal endometriosis and deep infiltrating endometriosis (Horne and Missmer 2022). OEM is more common in premenopausal women than in postmenopausal women (Gorp et al. 2004), and over 50% of patients with OEM had symptoms such as pelvic pain in their adolescences (Becker et al. 2022). Malignancy was the most fatal comorbidity for patients with OEM and OEM had a 0.9–4.5% probability of malignant transformation to epithelial ovarian cancer (EOC) (Gorp et al. 2004), that is, endometriosis associated ovarian cancer (EAOC).
Compared to OEM, EAOC showed no obvious symptoms (Lheureux et al. 2019), so identifying the occurrence of EAOC in OEM patients during long-term follow-up was crucial. Researchers found clinical characteristics of EAOC differed from those of OEM, such as age at diagnosis older, tumor larger and elevated levels of serum tumor markers (Hermens et al. 2020; Younis and Izhaki 2023; Murakami et al. 2020). We found that patients with EAOC had elevated serum cancer antigen (CA) 19–9 and human epididymis protein 4 (HE4) in a previous study (Xu et al. 2023).
In this study, we enrolled a larger sample size and involved more clinical characteristics extensively. Employing least absolute shrinkage and selection operator (LASSO) regression and Logistic regression, we derived a nomogram to diagnose EAOC.
Methods
Study design
Our study was a retrospective, single centered, case control study based on electronic medical record system of our institution. The study had been approved by Institutional Review Board (Ethnics Committee of First Affiliated Hospital of Nanjing Medical University, date: 2020-10-12, ID: 2020-MD-371), while written informed consents were exempted for retrospective study.
Study patients
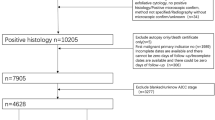
Among 1020 patients diagnosed with EOC from January 1, 2011 to January 1, 2023 in our institution, we selected patients in accord with diagnostic standard of EAOC. Classical diagnostic standard of EAOC was first proposed by Sampson (1925) and later supplemented by Scott (1953). Firstly, cancer and EM coexisted in the same ovary. Secondly, the cancer arose from EM other than another site. Thirdly, glands and surrounding stroma coexisted in EM. Fourthly, morphological continuity was found between cancer and EM. However, more and more researchers found this diagnostic standard might be too strict to cause missed diagnosis (Younis and Izhaki 2023; Murakami et al. 2020; Kawahara et al. 2022; Zhu et al. 2021; Kawahara et al. 2021; Similä-Maarala et al. 2022; Chao et al. 2022; Hernández et al. 2022). According to Gorp et al. (2004), diagnostic standard of EAOC in this study was EOC with pelvic EM. We expelled 185 patients for without surgeries, 739 patients without pelvic EM proven by pathologic examination, and 9 with dominant clinical characteristics incomplete. Finally, 87 patients with EAOC were enrolled in study group (Fig. 1).
The control group of the study was selected from patients diagnosed with OEM in the same period as EAOC patients in our institution. To compensating for limitation of sample size of EAOC group, we enlarged fourfold the OEM group to 348 cases randomly selected from 5034 patients with OEM. A total of 435 patients composed the study population, and 70% of the patients (N = 304) were randomly sampled as derivation set, while the remainders (N = 131) were involved in validation set (Fig. 1).
Data collection
Clinical characteristics of the patients were collected from their electronic medical records. Preoperative examinations were carried out within one week before surgeries. These characteristics covered demographics (age at diagnosis and body mass index (BMI)), reproductive and medical history (gravidity, parity, tumor size, previous abdominal surgery, hypertension, diabetes, other malignancy, uterine leiomyoma and other benign ovarian tumor), menstruation (dysmenorrhea and menopausal status), serologic tumor markers, white blood cell count and classification, coagulation function and blood biochemistry examination. Risk of ovarian malignancy algorithm (ROMA), deriving from CA125, HE4 and menopausal status, was proposed as a model assisting diagnosis of epithelial ovarian cancer (Gentry-Maharaj et al. 2020; Suri et al. 2021). Copenhagen index (CPH-I), calculated with age, CA125 and HE4 was another diagnostic model for indicating malignancy of adnexal masses (Karlsen et al. 2015). As inflammatory composite markers, monocyte lymphocyte ratio (MLR) and neutrophil lymphocyte ratio (NLR) were used to study diagnosis and prognosis of OC (Zhang et al. 2023; Leng et al. 2022). Thus, we calculated above indicators to analyze clinical characteristics of these patients comprehensively.
Statistical analysis
Continuous variates fitting and not fitting normal distribution were described as means with standard deviations and medians with quartile ranges, respectively, while categorical variables were described as counts and proportions. To comparing differences between clinical characteristics of patients with EAOC and OEM, t-tests were used for normally distributed continuous variates, while rank sum tests were used for non-normally distributed continuous variates and rank variates. Chi-square tests or Fisher’s exact tests were used for categorical variables.
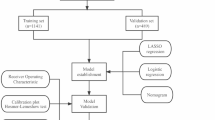
LASSO regression was employed to select variables included in prediction model, and multivariate Logistic regression were used to build model. To examine stability of model in derivation set, a bootstrap resampling with 1000 repetitions was performed. Receiver operating characteristic analyses (ROCs) and areas under curves (AUCs) were used to estimate discrimination sensitivities of the model, while calibration curves were employed to evaluate comparisons between predicted and actual diagnoses in derivation and validation sets. Decision curve analyses (DCAs) were performed to show clinical benefit of the model. The model was visualized as nomogram and web-based dynamic nomogram to be easily used in clinical practice.
Statistical analyses were performed using R software version 4.1.2 and Stata SE version 15. All statistical comparisons were two-sided, and differences were significant at P < 0.05.
Results
Clinical characteristics of patients with EAOC and OEM
Compared to the OEM group, patients in the EAOC group were older (EAOC vs. OEM: 46.41 ± 9.62 vs. 36.49 ± 8.09 year, P < 0.001) and had higher BMIs (23.45 ± 2.97 vs. 22.01 ± 3.18 kg/m2, P < 0.001). Additionally, patients with EAOC had higher times of pregnancies (2 (1–3) vs. 1 (0–1), P = 0.002) and deliveries (1 (1–1) vs. 1 (0–1), P < 0.001) compared to patients with OEM. Furthermore, EAOC patients were more likely to be postmenopausal women (30 (34.5%) vs. 5 (1.4%), P < 0.001) and had higher proportions of individuals with hypertension (13 (14.9%) vs. 10 (2.9%), P < 0.001), diabetes (3 (3.4%) vs. 2 (0.6%), P = 0.092) and other malignancies (8 (9.2%) vs. 15 (4.3%), P = 0.120). Conversely, a higher proportion of OEM patients experienced dysmenorrhea (25 (28.7%) vs. 218 (62.6%), P < 0.001), and the detection rates of uterine leiomyoma (11 (12.6%) vs. 135 (38.8%), P < 0.001) and other ovarian benign tumors (1 (1.1%) vs. 29 (8.3%), P = 0.018) were also higher in this group (Table 1).
Further analysis revealed distinct differences in the results of preoperative blood tests between the patients in the two groups. Patients with EAOC exhibited higher levels of CA125, CA19-9, HE4, and ROMA compared to patients with OEM. Additionally, these patients had elevated levels of white blood cell counts and higher percentages of neutrophils, while their percentages of lymphocytes were lower, resulting in higher MLRs and NLRs. Moreover, the EAOC group showed higher levels of fibrinogen (FIB) and d-dimer (DD2), which suggests more severe states of hypercoagulability. Contrastingly, patients with EAOC had lower levels of high-density lipoprotein (HDL) and low-density lipoprotein (LDL), as well as lower levels of total protein (TP) and albumin globulin ratio (AGR), indicating poorer nutritional statuses (Table 1).
Among 87 patients with EAOC, histological type of 46 (52.9%) patients was clear cell carcinoma, that of 31 (35.6%) patients was endometrioid carcinoma, that of seven (8.0%) patients was serous carcinoma and that of three (3.4%) patients was mucinous carcinoma (Table 1).
Development of prediction model
The derivation set consisted of 59 patients with EAOC and 245 patients with OEM, and the characteristics of 304 patients were used to construct the prediction model. LASSO regression was used to screen variables, and Logistic regression was used to construct the model. All variables were entered into LASSO regression with tenfold cross-validation, and at the lambda value equal to 0.06882337, variables with nonzero coefficients, including age, postmenopausal, tumor size, CA19-9 and ROMA (Fig. 2).
Since menopausal status played a role in calculating ROMA, to minimize the interaction effect, we excluded postmenopausal from construction of the model. Finally, a four-variable prediction model was constructed using age, tumor size, CA19-9 and ROMA. Older age, larger tumor size, higher CA19-9 and higher ROMA were identified as independent risk factors (Table 2).
Performance of prediction model
The performance of the predictive model was assessed in both the derivation and validation sets. ROC analysis and calibration curves were employed to evaluate the accuracy and agreement of the model in diagnosing EAOC, while DCA was utilized to assess the clinical utility of the model. In the training set, the AUC value for diagnosing EAOC was 0.858 (95% CI 0.795–0.920) (Fig. 3). The adjusted AUC value, obtained through 1000 repeated bootstrap resampling, was also 0.855 (95% CI 0.788–0.916), showing good consistency in the derivation set. The calibration curve demonstrated a good fit of the model in the training set (Hosmer–Lemeshow (HL) test chi-square = 12.600, P = 0.247). According to the DCA curve, this model exhibited superior clinical benefits over CPH-I within the threshold range of 0.08–0.92. Furthermore, our model showed superior performance compared to CPH-I in diagnosing EAOC, as evidenced by a higher AUC value (EAOC vs. OEM: 0.858 vs. 0.746, Delong test Z = 3.093, P = 0.002) and more favorable sensitivity (71.19% vs. 55.93%) and specificity (93.47% vs. 82.04%) (Table S2). Similarly, in the validation set, the ROC curve showed an AUC value of 0.870 (95% CI: 0.779–0.961) for the model's diagnostic performance in EAOC. The calibration curve indicated a good fit of the model in the training set (HL test chi-square = 8.210, P = 0.608), and the DCA curve demonstrated that the threshold range for clinical benefit was between 0.07 and 0.83, favoring this model over CPH-I (Fig. 3).
Visualization of prediction model
To facilitate the practical application of the predictive model, we presented it in the form of a nomogram (Fig. 4) and a web-based dynamic nomogram (https://tingxu-1.shinyapps.io/dynnomapp/). For instance, let’s consider the first patient in the validation set, the patient's age of 32 years corresponded to 16.8 points, lesion size of 10 cm corresponded to 30.6 points, CA19-9 level of 1.99 corresponded to 22.4 points, ROMA value of 2.88 corresponded to 15.8 points, resulting in a total score of 85.6 points. Consequently, the probability of diagnosing EAOC for this patient was calculated as 0.0533, and the actual diagnosis was OEM.
Discussion
EAOC is a unique type of EOC that arises from endometriosis. OEM is a benign disease with high prevalence and recurrence rates (Allaire et al. 2023), and was often diagnosed at a young age (Nnoaham et al. 2011). Consequently, a large number of patients with OEM required long-term managements, making EAOC a rare but life-threatening complication that affected these women. As a subtype of EOC, EAOC mostly lacked typical symptoms and was hard to diagnose before surgery (Younis and Izhaki 2023). In this study, we found that the proportion of EAOC patients with symptoms such as dysmenorrhea was lower compared to those with OEM. Therefore, an effective and convenient method for early diagnosis of EAOC is an urgent issue in the long-term management of OEM.
Since the first report by Sampson (1925), extensive research has been conducted worldwide to investigate and understand special features of EAOC. Through a systematic review of previous study, Younis et al. found that 70% of patients diagnosed with EAOC were over the age of 50, and a tumor size larger than 9 cm was identified as a risk factor for EAOC (Younis and Izhaki 2023). Phung et al. (2022) revealed that EAOC patients might have a higher BMI compared to patients with OEM. Chao et al. (2022) discovered that the significance of CA125 and CA19-9 in diagnosing EAOC. Our previous study found that age at diagnosis over 42 years, tumor size over 9.2 cm, and elevated CA19-9 and HE4 levels are risk factors for EAOC (Xu et al. 2023). In this study, we expanded the population and further validated these findings.
EAOC exhibited distinct clinical characteristics compared to OEM, highlighting the need for a portable and effective preoperative diagnostic model using machine learning. Chao et al. (2022) constructed a diagnostic model for EAOC using gradient decision trees and demonstrated its favorable performance. However, the drawback of this model was its reliance on a computer, which made it inconvenient and limited its widespread usage. To address this limitation, our study employed LASSO regression to select variables and Logistic regression to construct the model. The model was presented and utilized in the form of nomogram, offering ease of use and potential assistance to clinicians in diagnosing EAOC. Our prediction model incorporated age, tumor size, CA19-9, and ROMA as variates. Furthermore, we assessed the model's performance in both the training and validation sets using ROC analysis, calibration curves, and DCA, and found that it performed well.
Our study successfully developed an easy-to-use model for preoperative diagnosis of EAOC. Additionally, we employed machine learning-based LASSO regression and Logistic regression to reduce confounding bias in this retrospective observational study. To minimize selection bias, simple random sampling was used in selecting control groups. However, the study had limitations, including a small sample size and the presence of unavoidable information bias. Additionally, absence of an external validation cohort could limit our prediction model widely used. In order to address these limitations, we plan to collaborate with domestic and international research groups for further study.
In this study, we studied clinical characteristics of patients with EAOC and developed a preoperative diagnostic model that incorporated age, tumor size, CA19-9, and ROMA. We found that this model has a favorable performance in our cohort and was potential to aid clinicians in early diagnosis of EAOC.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Allaire C, Bedaiwy MA, Yong PJ (2023) Diagnosis and management of endometriosis. CMAJ 195:E363–E371. https://doi.org/10.1503/cmaj.220637
Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, King K, Kvaskoff M, Nap A, Petersen K, Saridogan E, Tomassetti C, van Hanegem N, Vulliemoz N, Vermeulen N (2022) ESHRE guideline: endometriosis. Hum Reprod Open 2022:hoac009. https://doi.org/10.1093/hropen/hoac009
Chao X, Wang S, Lang J, Leng J, Fan Q (2022) The application of risk models based on machine learning to predict endometriosis-associated ovarian cancer in patients with endometriosis. Acta Obstet Gynecol Scand 101:1440–1449. https://doi.org/10.1111/aogs.14462
Gentry-Maharaj A, Burnell M, Dilley J, Ryan A, Karpinskyj C, Gunu R, Mallett S, Deeks J, Campbell S, Jacobs I, Sundar S, Menon U (2020) Serum HE4 and diagnosis of ovarian cancer in postmenopausal women with adnexal masses. Am J Obstet Gynecol 222:561–56 e17. https://doi.org/10.1016/j.ajog.2019.07.031
Gorp TV, Amant F, Neven P, Vergote I, Moerman P (2004) Endometriosis and the development of malignant tumours of the pelvis. A review of literature. Best Pract Res Clin Obstet Gynaecol 18:349–371. https://doi.org/10.1016/j.bpobgyn.2003.03.001
Hermens M, van Altena AM, Nieboer TE, Schoot BC, van Vliet HAAM, Siebers AG, Bekkers RLM (2020) Incidence of endometrioid and clear-cell ovarian cancer in histological proven endometriosis: the ENOCA population-based cohort study. Am J Obstet Gynecol 223:107 e1-107 e11. https://doi.org/10.1016/j.ajog.2020.01.041
Hernández A, Sanz A, Spagnolo E, Carbonell M, Rodríguez E, López A, Raganato R, Del Forno S, Ramiro-Cortijo D (2022) Evaluation of women’s age and ultrasonographic features to choose surgical treatment for endometriosis associated with ovarian cancer. J Clin Med 11:2414. https://doi.org/10.3390/jcm11092414
Horne AW, Missmer SA (2022) Pathophysiology, diagnosis, and management of endometriosis. BMJ 379:070750. https://doi.org/10.1136/bmj-2022-070750
Karlsen MA, Høgdall EV, Christensen IJ, Borgfeldt C, Kalapotharakos G, Zdrazilova-Dubska L, Chovanec J, Lok CA, Stiekema A, Mutz-Dehbalaie I, Rosenthal AN, Moore EK, Schodin BA, Sumpaico WW, Sundfeldt K, Kristjansdottir B, Zapardiel I, Høgdall CK (2015) A novel diagnostic index combining HE4, CA125 and age may improve triage of women with suspected ovarian cancer—an international multicenter study in women with an ovarian mass. Gynecol Oncol 138:640–646. https://doi.org/10.1016/j.ygyno.2015.06.021
Kawahara N, Miyake R, Yamanaka S, Kobayashi H (2021) A novel predictive tool for discriminating endometriosis associated ovarian cancer from ovarian endometrioma: the R2 predictive index. Cancers (basel) 13:3829. https://doi.org/10.3390/cancers13153829
Kawahara N, Kawaguchi R, Maehana T, Yamanaka S, Yamada Y, Kobayashi H, Kimura F (2022) The endometriotic neoplasm algorithm for risk assessment (e-NARA) index sheds light on the discrimination of endometriosis-associated ovarian cancer from ovarian endometrioma. Biomedicines 10:2683. https://doi.org/10.3390/biomedicines10112683
Leng J, Wu F, Zhang L (2022) Prognostic significance of pretreatment neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, or monocyte-to-lymphocyte ratio in endometrial neoplasms: a systematic review and meta-analysis. Front Oncol 12:734948. https://doi.org/10.3389/fonc.2022.734948
Lheureux S, Gourley C, Vergote I, Oza AM (2019) Epithelial ovarian cancer. Lancet 393:1240–1253. https://doi.org/10.1016/S0140-6736(18)32552-2
Murakami K, Kotani Y, Nakai H, Matsumura N (2020) Endometriosis-associated ovarian cancer: the origin and targeted therapy. Cancers (basel) 12:1676. https://doi.org/10.3390/cancers12061676
Nnoaham KE, Hummelshoj L, Webster P, d’Hooghe T, de Cicco NF, de Cicco NC, Jenkinson C, Kennedy SH, Zondervan KT (2011) Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 96:366–373. https://doi.org/10.1016/j.fertnstert.2011.05.090
Phung MT, Muthukumar A, Trabert B, Webb PM, Jordan SJ, Terry KL, Cramer DW, Titus LJ, Risch HA, Doherty JA, Harris HR, Goodman MT, Modugno F, Moysich KB, Jensen A, Kjaer SK, Anton-Culver H, Ziogas A, Berchuck A, Khoja L, Wu AH, Pike MC, Pearce CL, Lee AW (2022) Effects of risk factors for ovarian cancer in women with and without endometriosis. Fertil Steril 118:960–969. https://doi.org/10.1016/j.fertnstert.2011.05.090
Sampson JA (1925) Endometrial carcinoma of ovary arising in endometrial tissue in that organ. Arch Surg 10:1–72
Scott RB (1953) Malignant changes in endometriosis. Obstet Gynecol 2:283–289
Similä-Maarala J, Soovares P, Pasanen A, Ahvenainen T, Vahteristo P, Bützow R, Lassus H (2022) TCGA molecular classification in endometriosis-associated ovarian carcinomas: novel data on clear cell carcinoma. Gynecol Oncol 165:577–584. https://doi.org/10.1016/j.ygyno.2022.03.016
Suri A, Perumal V, Ammalli P, Suryan V, Bansal SK (2021) Diagnostic measures comparison for ovarian malignancy risk in epithelial ovarian cancer patients: a meta-analysis. Sci Rep 11:17308. https://doi.org/10.1038/s41598-021-96552-9
Xu T, Zhang L, Jiang Y, Wan Y, Zhou S, Sun G, Cheng W, Yuan L (2023) Clinical characteristics and serum CA19-9 combined with HE4 are valuable in diagnosing endometriosis-associated ovarian cancer. Int J Gynaecol Obstet 162:472–478. https://doi.org/10.1002/ijgo.14695
Younis JS, Izhaki I (2023) At what age endometriosis-associated ovarian cancer is diagnosed? The implications for women in the reproductive age. Front Oncol 13:1193123. https://doi.org/10.3389/fonc.2023.1193123
Zhang CL, Jiang XC, Li Y, Pan X, Gao MQ, Chen Y, Pang B (2023) Independent predictive value of blood inflammatory composite markers in ovarian cancer: recent clinical evidence and perspective focusing on NLR and PLR. J Ovarian Res 16:36. https://doi.org/10.1186/s13048-023-01116-2
Zhu C, Zhu J, Qian L, Liu H, Shen Z, Wu D, Zhao W, Xiao W, Zhou Y (2021) Clinical characteristics and prognosis of ovarian clear cell carcinoma: a 10-year retrospective study. BMC Cancer 21:322. https://doi.org/10.1186/s12885-021-08061-7
Zondervan KT, Becker CM, Missmer SA (2020) Endometriosis. N Engl J Med 382:1244–1256. https://doi.org/10.1056/NEJMra1810764
Acknowledgements
This study was supported by the National Natural Science Foundation of China, National Nature Science Foundation for young scientist, National Nature Science Foundation for young scientist in Jiangsu Province, Jiangsu Province 333 high level talent funds, Jiangsu Province Traditional Chinese Medicine Science and Technology Development Plan Project-Key Project, General Project of Yili Clinical Medical Research Institute and Jiangsu Provincial Maternal and Child Health Research Fund.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81872119), National Nature Science Foundation for young scientist (No. 82103286), National Nature Science Foundation for young scientist in Jiangsu Province (No. BK20210971), Jiangsu Province 333 high level talent funds (2019), Jiangsu Province Traditional Chinese Medicine Science and Technology Development Plan Project-Key Project (No. ZD202014), General Project of Yili Clinical Medical Research Institute (2022). Jiangsu Provincial Maternal and Child Health Research Fund (2022).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by TX, LZ and YJ. Data collection and analyses were performed by XN, HM and YW. The first draft of the manuscript was written by TX and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethnics Committee of First Affiliated Hospital of Nanjing Medical University (2020-10-12/ID: 2020-MD-371).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, T., Nie, X., Zhang, L. et al. Derivation and validation of a nomogram based on clinical characteristics to diagnose endometriosis associated ovarian cancer preoperatively. J Cancer Res Clin Oncol 150, 19 (2024). https://doi.org/10.1007/s00432-023-05524-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-023-05524-1