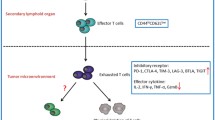
Abstract
Liver cancer is one of the most common malignancies. T-cell exhaustion is associated with immunosuppression of tumor and chronic infection. Although immunotherapies that enhance the immune response by targeting programmed cell death-1(PD-1)/programmed cell death ligand 1 (PD-L1) have been applied to malignancies, these treatments have shown limited response rates. This suggested that additional inhibitory receptors (IRs) also contributed to T-cell exhaustion and tumor prognosis. Exhausted T-cells (Tex) in the tumor immune microenvironment (TME) are usually in a dysfunctional state of exhaustion, such as impaired activity and proliferative ability, increased apoptosis rate, and reduced production of effector cytokines. Tex cells participate in the negative regulation of tumor immunity mainly through IRs on the cell surface, changes in cytokines and immunomodulatory cell types, causing tumor immune escape. However, T-cell exhaustion is not irreversible and targeted immune checkpoint inhibitors (ICIs) can effectively reverse the exhaustion of T-cells and restore the anti-tumor immune response. Therefore, the research on the mechanism of T-cell exhaustion in liver cancer, aimed at maintaining or restoring the effector function of Tex cells, might provide a new method for the treatment of liver cancer. In this review, we summarized the basic characteristics of Tex cells (such as IRs and cytokines), discussed the mechanisms associated with T-cell exhaustion, and specifically discussed how these exhaustion characteristics were acquired and shaped by key factors within TME. Then new insights into the molecular mechanism of T-cell exhaustion suggested a potential way to improve the efficacy of cancer immunotherapy, namely to restore the effector function of Tex cells. In addition, we also reviewed the research progress of T-cell exhaustion in recent years and provided suggestions for further research.





Similar content being viewed by others
Abbreviations
- MTA:
-
5-Methylthioadenosine
- ADORA1:
-
Adenosine A1 receptor
- AGTRAP:
-
Angiotensin II receptor-associated protein
- APCs:
-
Antigen-presenting cells
- ARG1:
-
Arginase I
- CEACAM-1:
-
Carcinoembryonic antigen-related cell adhesion molecule 1
- CHB:
-
Chronic hepatitis B
- CSF-1:
-
Colony stimulating factor 1
- COX-2:
-
Cyclooxygenase-2
- CTLA-4:
-
Cytotoxic T lymphocyte-associated antigen-4
- CTLs:
-
Cytotoxic T lymphocytes
- Eomes:
-
Eomesodermin
- Tex:
-
Exhausted T-cells
- eADO:
-
Extracellular adenosine
- FGL-1:
-
Fibrinogen-like protein 1
- Gal-9:
-
Galactose lectin 9
- GLIS1:
-
GLI-similar 1
- GPNMB:
-
Glycoprotein nonmetastatic melanoma protein B
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- HCC:
-
Hepatocellular carcinoma
- HMGB1:
-
High mobility group box-1 protein
- ICIs:
-
Immune checkpoint inhibitors
- ICIs:
-
Immune checkpoint inhibitors
- IRs:
-
Inhibitory receptors
- IRF 8:
-
Interferon regulatory factor 8
- IFN-γ:
-
Interferon-γ
- IL-2:
-
Interleukin-2
- KPNA4:
-
Karyopherin α4
- LAYN:
-
Layilin
- LAG-3:
-
Lymphocyte-activation gene 3
- LCMV:
-
Lymphocytic choriomeningitis virus
- MDZ:
-
Midazolam
- MRS:
-
Myeloid response scores
- MDSCs:
-
Myeloid-derived suppressor cells
- NETs:
-
Neutrophil extracellular traps
- NASH:
-
Non-alcoholic steatohepatitis
- NTME:
-
Nontumor microenvironment
- NuRD:
-
Nucleosome remodeling and deacetylase
- PtdSer:
-
Phosphatidylserine
- pDCs:
-
Plasmacytoid dendritic cells
- PD-L1:
-
Programmed cell death ligand 1
- PD-1:
-
Programmed cell death-1
- PGE-2:
-
Prostaglandin E-2
- PTP1B:
-
Protein-tyrosine phosphatase 1B
- RBPJ:
-
Recombination signal binding protein for immunoglobulin kappa J region
- Tregs:
-
Regulatory T-cell
- SAM:
-
S-adenosylmethionine
- sTIM-3:
-
Soluble TIM-3
- SPAG5:
-
Sperm-associated antigen 5
- TBK1:
-
TANK-Binding Kinase 1
- TIGIT:
-
T-cell Ig and ITIM domain
- TIM-3:
-
T-cell immunoglobulin and mucin-domain containing-3
- TOX:
-
Thymocyte selection-associated high mobility group-box protein
- TGF-β1:
-
Transforming growth factor beta 1
- TECs:
-
Tumor endothelial cells
- TME:
-
Tumor immune microenvironment
- TNF-α:
-
Tumor necrosis factor-α
- TAMs:
-
Tumor-associated macrophages
- TANs:
-
Tumor-associated neutrophils
- TILs:
-
Tumor-infiltrating lymphocyte
- TST:
-
Tumor-specific CD8+T-cells
- XDH:
-
Xanthine dehydrogenase
- YTHDF2:
-
YTH domain family 2
References
Andrews LP, Marciscano AE, Drake CG, Vignali DA (2017) LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev 276:80–96
Bai X et al (2022) Adaptive antitumor immune response stimulated by bio-nanoparticle based vaccine and checkpoint blockade. J Exp Clin Cancer Res 41:022–02307
Bao S, Jiang X, Jin S, Tu P, Lu J (2021) TGF-β1 Induces immune escape by enhancing PD-1 and CTLA-4 expression on T lymphocytes in hepatocellular carcinoma. Front Oncol 11.
Barsch M et al (2022) T-cell exhaustion and residency dynamics inform clinical outcomes in hepatocellular carcinoma. J Hepatol 77:397–409
Buggert M et al. (2014) T-bet and Eomes are differentially linked to the exhausted phenotype of CD8+ T cells in HIV infection. PLoS Pathog 10.
Cai L et al (2008) Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol 129:428–437
Cham CM, Gajewski TF (2005) Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J Immunol 174:4670–4677
Chang CH et al (2015) Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162:1229–1241
Chauvin JM, Zarour HM (2020) TIGIT in cancer immunotherapy. J Immunother Cancer 8:2020–000957
Chen DS, Mellman I (2013) Oncology meets immunology: the cancer-immunity cycle. Immunity 39:1–10
Chen Z et al (2020a) Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res 10:2993–3036
Chen W, Chen X, Li S, Ren B (2020b) Expression, immune infiltration and clinical significance of SPAG5 in hepatocellular carcinoma: a gene expression-based study. J Gene Med 22:27
Cheng Y et al (2021) Non-terminally exhausted tumor-resident memory HBV-specific T cell responses correlate with relapse-free survival in hepatocellular carcinoma. Immunity 54:1825–1840
Chew V et al (2017) Delineation of an immunosuppressive gradient in hepatocellular carcinoma using high-dimensional proteomic and transcriptomic analyses. Proc Natl Acad Sci USA 114:E5900–E5909
Cui S et al. (2023) Development of prognostic features of hepatocellular carcinoma based on metabolic gene classification and immune and oxidative stress characteristic analysis. Oxid Med Cell Longev 18.
Deng Z et al (2021) Key candidate prognostic biomarkers correlated with immune infiltration in hepatocellular carcinoma. J Hepatocell Carcinoma 8:1607–1622
Dong P et al. (2016) CD86+/CD206+, diametrically polarized tumor-associated macrophages, predict hepatocellular carcinoma patient prognosis. Int J Mol Sci 17.
EASL Clinical Practice Guidelines (2018) Management of hepatocellular carcinoma. J Hepatol 69:182–236
Facciabene A et al (2011) Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 475:226–230
Fisicaro P et al (2017) Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat Med 23:327–336
Fletcher M et al (2015) l-Arginine depletion blunts antitumor T-cell responses by inducing myeloid-derived suppressor cells. Cancer Res 75:275–283
Fourcade J et al (2010) Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 207:2175–2186
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9:162–174
Gao Y et al (2022) Heterogeneity induced GZMA-F2R communication inefficient impairs antitumor immunotherapy of PD-1 mAb through JAK2/STAT1 signal suppression in hepatocellular carcinoma. Cell Death Dis 13:022–04654
Garrido F, Cabrera T, Aptsiauri N (2010) “Hard” and “soft” lesions underlying the HLA class I alterations in cancer cells: implications for immunotherapy. Int J Cancer 127:249–256
Ge Z et al (2021) TIGIT and PD1 Co-blockade restores ex vivo functions of human tumor-infiltrating CD8(+) T cells in hepatocellular carcinoma. Cell Mol Gastroenterol Hepatol 12:443–464
Gehring AJ et al (2009) Profile of tumor antigen-specific CD8 T cells in patients with hepatitis B virus-related hepatocellular carcinoma. Gastroenterology 137:682–690
Ghoneim HE et al (2017) De novo epigenetic programs inhibit PD-1 blockade-mediated T cell rejuvenation. Cell 170:142–157
Giraud J, Chalopin D, Blanc JF, Saleh M (2021) Hepatocellular carcinoma immune landscape and the potential of immunotherapies. Front Immunol 12.
Gu Y et al (2020) CCL14 is a prognostic biomarker and correlates with immune infiltrates in hepatocellular carcinoma. Aging 12:784–807
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
He H et al (2021) Down-regulation of EOMES drives T-cell exhaustion via abolishing EOMES-mediated repression of inhibitory receptors of T cells in liver cancer. J Cell Mol Med 25:161–169
He J, Meng M, Wang H (2022) A novel prognostic biomarker LPAR6 in hepatocellular carcinoma via associating with immune infiltrates. J Clin Transl Hepatol 10:90–103
Hsu CL et al (2021) Exploring markers of exhausted CD8 T cells to predict response to immune checkpoint inhibitor therapy for hepatocellular carcinoma. Liver Cancer 10:346–359
Hung MH et al (2021) Tumor methionine metabolism drives T-cell exhaustion in hepatocellular carcinoma. Nat Commun 12:021–21804
Ji J et al (2018) Long non-coding RNA Lnc-Tim3 exacerbates CD8 T cell exhaustion via binding to Tim-3 and inducing nuclear translocation of Bat3 in HCC. Cell Death Dis 9:018–0528
Jiang Y et al. (2021) TANK-Binding Kinase 1 (TBK1) Serves as a Potential target for hepatocellular carcinoma by enhancing tumor immune infiltration. Front Immunol 12.
Jin H et al (2022a) New insights into checkpoint inhibitor immunotherapy and its combined therapies in hepatocellular carcinoma: from mechanisms to clinical trials. Int J Biol Sci 18:2775–2794
Jin Y et al (2022b) Expression of Id3 represses exhaustion of anti-tumor CD8 T cells in liver cancer. Mol Immunol 144:117–126
Johnston RJ et al (2014) The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 26:923–937
Kaech SM, Cui W (2012) Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 12:749–761
Kaltenmeier C et al. (2021) Neutrophil extracellular traps promote T cell exhaustion in the tumor microenvironment. Front Immunol 12.
Kang J et al (2022) Midazolam exhibits antitumour and enhances the efficiency of Anti-PD-1 immunotherapy in hepatocellular carcinoma. Cancer Cell Int 22:022–02735
Kato Y et al. (2019) Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One 14.
Kelly C et al (2016) Chronic hepatitis C viral infection subverts vaccine-induced T-cell immunity in humans. Hepatology 63:1455–1470
Khan M, Arooj S, Wang H (2020) NK cell-based immune checkpoint inhibition. Front Immunol 11.
Kim HD et al (2020) 4–1BB delineates distinct activation status of exhausted tumor-infiltrating CD8(+) T cells in hepatocellular carcinoma. Hepatology 71:955–971
Kitamura T, Qian BZ, Pollard JW (2015) Immune cell promotion of metastasis. Nat Rev Immunol 15:73–86
Kotanides H et al (2020) Bispecific targeting of PD-1 and PD-L1 enhances T-cell activation and antitumor immunity. Cancer Immunol Res 8:1300–1310
Kučan Brlić, P et al. (2019) Targeting PVR (CD155) and its receptors in anti-tumor therapy. Cell Mol Immunol 16: 40–52.
Kumar V, Patel S, Tcyganov E, Gabrilovich DI (2016) The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol 37:208–220
Laidlaw BJ, Craft JE, Kaech SM (2016) The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat Rev Immunol 16:102–111
Lawal G et al. (2021) The Immunology of Hepatocellular Carcinoma. Vaccines 9.
Li Z et al (2012a) TIM3 gene polymorphisms in patients with chronic hepatitis B virus infection: impact on disease susceptibility and hepatocellular carcinoma traits. Tissue Antigens 80:151–157
Li T et al (2012b) Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett 318:154–161
Li F et al (2018a) Highly elevated soluble Tim-3 levels correlate with increased hepatocellular carcinoma risk and poor survival of hepatocellular carcinoma patients in chronic hepatitis B virus infection. Cancer Manag Res 10:941–951
Li J et al (2018b) Co-inhibitory molecule B7 superfamily member 1 expressed by tumor-infiltrating myeloid cells induces dysfunction of anti-tumor CD8(+) T cells. Immunity 48:773–786
Li Q et al (2022) PRDM1/BLIMP1 induces cancer immune evasion by modulating the USP22-SPI1-PD-L1 axis in hepatocellular carcinoma cells. Nat Commun 13:022–35469
Lim CJ et al (2019) Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut 68:916–927
Lin Z et al (2021) Xanthine dehydrogenase as a prognostic biomarker related to tumor immunology in hepatocellular carcinoma. Cancer Cell Int 21:021–02173
Linsley PS et al (1991) Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med 173:721–730
Liu Y, Zheng P (2020) Preserving the CTLA-4 checkpoint for safer and more effective cancer immunotherapy. Trends Pharmacol Sci 41:4–12
Liu YT et al (2018) A novel spontaneous hepatocellular carcinoma mouse model for studying T-cell exhaustion in the tumor microenvironment. J Immunother Cancer 6:018–0462
Liu X et al (2019a) PD-1(+) TIGIT(+) CD8(+) T cells are associated with pathogenesis and progression of patients with hepatitis B virus-related hepatocellular carcinoma. Cancer Immunol Immunother 68:2041–2054
Liu B et al (2019b) The lineage stability and suppressive program of regulatory T cells require protein O-GlcNAcylation. Nat Commun 10:019–08300
Liu X, Ren H, Guo H, Wang W, Zhao N (2021) Interleukin-35 has a tumor-promoting role in hepatocellular carcinoma. Clin Exp Immunol 203:219–229
Liu F et al. (2020) Heterogeneity of exhausted T cells in the tumor microenvironment is linked to patient survival following resection in hepatocellular carcinoma. Oncoimmunology 9.
Liu SC et al. (2020) Research progress of CD8+T cell exhaustion mechanism and immunotherapy in hepatocellular carcinoma. Journal of Mudanjiang Medical University 41: 97-100, https://doi.org/10.13799/j.cnki.mdjyxyxb.2020.04.026 (2020).(in Chinese)
Liu S et al. (2021) AGTRAP Is a Prognostic biomarker correlated with immune infiltration in hepatocellular carcinoma. Front Oncol 11.
Lonberg N, Korman AJ (2017) Masterful antibodies: checkpoint blockade. Cancer Immunol Res 5:275–281
Lu Y et al (2022) A single-cell atlas of the multicellular ecosystem of primary and metastatic hepatocellular carcinoma. Nat Commun 13:022–32283
Ma J et al (2019) PD1(Hi) CD8(+) T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J Immunother Cancer 7:019–0814
Mamrot J, Balachandran S, Steele EJ, Lindley RA (2019) Molecular model linking Th2 polarized M2 tumour-associated macrophages with deaminase-mediated cancer progression mutation signatures. Scand J Immunol 89:18
Nishida N et al (2020) Association between genetic and immunological background of hepatocellular carcinoma and expression of programmed cell death-1. Liver Cancer 9:426–439
Ostroumov D et al (2021) Transcriptome profiling identifies TIGIT as a marker of T-cell exhaustion in liver cancer. Hepatology 73:1399–1418
Ouyang FZ et al. (2016) Dendritic cell-elicited B-cell activation fosters immune privilege via IL-10 signals in hepatocellular carcinoma. Nat Commun 7.
Pan B et al (2023) Targeted inhibition of RBPJ transcription complex alleviates the exhaustion of CD8(+) T cells in hepatocellular carcinoma. Commun Biol 6:023–04521
Pang L et al (2021) Plasmacytoid dendritic cells recruited by HIF-1α/eADO/ADORA1 signaling induce immunosuppression in hepatocellular carcinoma. Cancer Lett 522:80–92
Patsoukis N et al. (2015) PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun 6.
Pauken KE, Wherry EJ (2015) Overcoming T cell exhaustion in infection and cancer. Trends Immunol 36:265–276
Qian BZ, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141:39–51
Reading JL et al (2018) The function and dysfunction of memory CD8(+) T cells in tumor immunity. Immunol Rev 283:194–212
Rong D et al (2023) GLIS1 intervention enhances anti-PD1 therapy for hepatocellular carcinoma by targeting SGK1-STAT3-PD1 pathway. J Immunother Cancer 11:2022–005126
Sakano Y et al (2022) Tumor endothelial cell-induced CD8(+) T-cell exhaustion via GPNMB in hepatocellular carcinoma. Cancer Sci 113:1625–1638
Saraiva M, Vieira P, O’Garra A (2020) Biology and therapeutic potential of interleukin-10. J Exp Med 217:20190418
Schietinger A et al (2016) Tumor-Specific T Cell Dysfunction Is a Dynamic Antigen-Driven Differentiation Program Initiated Early during Tumorigenesis. Immunity 45:389–401
Sen DR et al (2016) The epigenetic landscape of T cell exhaustion. Science 354:1165–1169
Shao XY, Dong J, Zhang H, Wu YS, Zheng L (2020) Systematic analyses of the role of the reader protein of N (6)-methyladenosine RNA methylation, YTH domain family 2, in liver hepatocellular carcinoma. Front Mol Biosci 7.
Shen Q et al (2017) Lipid metabolic characteristics of functionally exhausted CD8+ T cells in tumor microenvironment of hepatocellular carcinoma. Chin J Clin Med 24:514–518 ((in Chinese))
Sheng H et al (2020) ATR inhibitor AZD6738 enhances the antitumor activity of radiotherapy and immune checkpoint inhibitors by potentiating the tumor immune microenvironment in hepatocellular carcinoma. J Immunother Cancer 8:2019–000340
Simoni Y et al (2018) Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557:575–579
Stephen TL et al (2017) SATB1 expression governs epigenetic repression of PD-1 in tumor-reactive T cells. Immunity 46:51–64
Sun C et al (2018) Oncofetal gene SALL4 reactivation by hepatitis B virus counteracts miR-200c in PD-L1-induced T cell exhaustion. Nat Commun 9:018–03584
Sung H et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Tan S et al (2020) Tim-3 hampers tumor surveillance of liver-resident and conventional NK cells by disrupting PI3K signaling. Cancer Res 80:1130–1142
Tomiyama T et al (2022) Myeloid-derived suppressor cell infiltration is associated with a poor prognosis in patients with hepatocellular carcinoma. Oncol Lett 23:27
Topalian SL, Taube JM, Pardoll DM (2020) Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 367.
Twyman-Saint Victor C et al. (2015) Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520: 373–377.
Villanueva A (2019) Hepatocellular carcinoma. N Engl J Med 380:1450–1462
Wang K, Karin M (2015) Tumor-elicited inflammation and colorectal cancer. Adv Cancer Res 128:173–196
Wang X, Lu XJ, Sun B (2017) The pros and cons of dying tumour cells in adaptive immune responses. Nat Rev Immunol 17:31
Wang X et al (2018) 14-3-3ζ delivered by hepatocellular carcinoma-derived exosomes impaired anti-tumor function of tumor-infiltrating T lymphocytes. Cell Death Dis 9:017–0180
Wang X et al (2019a) TOX promotes the exhaustion of antitumor CD8(+) T cells by preventing PD1 degradation in hepatocellular carcinoma. J Hepatol 71:731–741
Wang X, He Q, Shen H, Lu XJ, Sun B (2019b) Genetic and phenotypic difference in CD8(+) T cell exhaustion between chronic hepatitis B infection and hepatocellular carcinoma. J Med Genet 56:18–21
Wang J et al (2019c) Fibrinogen-like protein 1 Is a major immune inhibitory ligand of LAG-3. Cell 176:334–347
Wang YG, Zheng DH, Shi M, Xu XM (2019d) T cell dysfunction in chronic hepatitis B infection and liver cancer: evidence from transcriptome analysis. J Med Genet 56:22–28
Wang D et al (2019e) Macrophage-derived CCL22 promotes an immunosuppressive tumor microenvironment via IL-8 in malignant pleural effusion. Cancer Lett 452:244–253
Wang X et al (2019f) Exosomes derived from exhausted CD8+ T cells impaired the anticancer function of normal CD8+ T cells. J Med Genet 56:29–31
Wang XC (2019) Study on the mechanism of CD8+T cell depletion in invasive liver cancer. Nanjing Medical University. (in Chinese)
Wherry EJ, Kurachi M (2015) Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15:486–499
Wolf Y, Anderson AC, Kuchroo VK (2020) TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol 20:173–185
Woo SR et al (2012) Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 72:917–927
Wu X et al (2019) Application of PD-1 Blockade in Cancer Immunotherapy. Comput Struct Biotechnol J 17:661–674
Wu C et al (2020) Myeloid signature reveals immune contexture and predicts the prognosis of hepatocellular carcinoma. J Clin Invest 130:4679–4693
Wu SY et al (2021a) Correlation of MKI67 with prognosis, immune infiltration, and T cell exhaustion in hepatocellular carcinoma. BMC Gastroenterol 21:021–01984
Wu M, Xia X, Hu J, Fowlkes NW, Li S (2021b) WSX1 act as a tumor suppressor in hepatocellular carcinoma by downregulating neoplastic PD-L1 expression. Nat Commun 12:021–23864
Wu H et al (2022) Hepatic interferon regulatory factor 8 expression suppresses hepatocellular carcinoma progression and enhances the response to anti-programmed cell death protein-1 therapy. Hepatology 76:1602–1616
Wu Y et al (2023) Blockade of T-cell receptor with Ig and ITIM domains elicits potent antitumor immunity in naturally occurring HBV-related HCC in mice. Hepatology 77:965–981
Xu M et al (2017) Interactions between interleukin-6 and myeloid-derived suppressor cells drive the chemoresistant phenotype of hepatocellular cancer. Exp Cell Res 351:142–149
Xu W, Atkins MB, McDermott DF (2020) Checkpoint inhibitor immunotherapy in kidney cancer. Nat Rev Urol 17:137–150
Xu M et al (2021) Value of KPNA4 as a diagnostic and prognostic biomarker for hepatocellular carcinoma. Aging 13:5263–5283
Xu Y et al. (2020) DHX37 Impacts Prognosis of hepatocellular carcinoma and lung adenocarcinoma through immune infiltration. J Immunol Res 30.
Xun X et al (2021) Cyclooxygenase-2 expressed hepatocellular carcinoma induces cytotoxic T lymphocytes exhaustion through M2 macrophage polarization. Am J Transl Res 13:4360–4375
Yang Y et al (2018) Crosstalk between hepatic tumor cells and macrophages via Wnt/β-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death Dis 9:018–0818
Yang Y et al (2020) Analysis of single-cell RNAseq identifies transitional states of T cells associated with hepatocellular carcinoma. Clin Transl Med 10:13
Yang R et al (2021) Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun 12:021–21099
Yang C et al. (2023) Fibrinogen-like protein 1 promotes liver-resident memory T-cell exhaustion in hepatocellular carcinoma. Front Immunol 14.
Yatim N, Cullen S, Albert ML (2017) Dying cells actively regulate adaptive immune responses. Nat Rev Immunol 17:262–275
Yazdani HO, Tohme S (2019) Murine model of metastatic liver tumors in the setting of ischemia reperfusion injury. J vis Exp 30:59748
Yin C et al. (2019) SALL4-mediated upregulation of exosomal miR-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in HCC. Oncoimmunology 8.
Yuan RL, Zheng MJ, Chen ZX, Xu YH (2021) TIM-3+TIGIT+CD4+T cell and TIM-3+TIGIT+CD8+T cell immune exhaustion in patients with hepatocellular carcinoma and its relationship with disease progression. Acta Universitatis Medicinalis Anhui 56: 1980–1986, https://doi.org/10.19405/j.cnki.issn1000-1492.2021.12.025.(in Chinese)
Zajac AJ et al (1998) Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 188:2205–2213
Zhang X et al (2004) Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity 20:337–347
Zhang J, Shi Z, Xu X, Yu Z, Mi J (2019) The influence of microenvironment on tumor immunotherapy. Febs J 286:4160–4175
Zhang C et al (2020a) TIGIT can exert immunosuppressive effects on CD8+ T cells by the CD155/TIGIT signaling pathway for hepatocellular carcinoma in vitro. J Immunother 43:236–243
Zhang W et al (2020b) IL-6 promotes PD-L1 expression in monocytes and macrophages by decreasing protein tyrosine phosphatase receptor type O expression in human hepatocellular carcinoma. J Immunother Cancer 8:2019–000285
Zhang P et al (2022) Neuregulin 4 suppresses NASH-HCC development by restraining tumor-prone liver microenvironment. Cell Metab 34:1359–1376
Zheng C et al (2017) Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell 169:1342–1356
Zhu Y et al (2022) The combination of PD-1 blockade with interferon-α has a synergistic effect on hepatocellular carcinoma. Cell Mol Immunol 19:726–737
Zhu Y, Zu Y (2023) Comprehensive bioinformatics analysis reveals PTPN1 (PTP1B) is a promising immunotherapy target associated with T cell function for liver cancer. J Healthc Eng 27.
Zou F et al (2021) The CD39(+) HBV surface protein-targeted CAR-T and personalized tumor-reactive CD8(+) T cells exhibit potent anti-HCC activity. Mol Ther 29:1794–1807
Acknowledgements
The authors acknowledge using Biorender (https://app.biorender.com/user/signin) to create the schemata (Figs. 2, 3).
Funding
The present study was financially supported by the National Natural Science Foundation of China (No. 81973840 and No. 81273748); National Science and Technology major projects of the 13th Five-Year Plan (2018ZX10303502); Science and Technology Program of Hebei (223777156D); Sichuan Provincial Administration of Traditional Chinese Medicine Major science and technology projects (2021XYCZ004).
Author information
Authors and Affiliations
Contributions
XH designed the research; LH and SL wrote the manuscript with contributions from all authors. All authors read and approved the initial manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Declarations
All authors read and approved the initial manuscript.
Conflcit of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors approved the final manuscript and the submission to this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hao, L., Li, S. & Hu, X. New insights into T-cell exhaustion in liver cancer: from mechanism to therapy. J Cancer Res Clin Oncol 149, 12543–12560 (2023). https://doi.org/10.1007/s00432-023-05083-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-05083-5