Abstract
Purpose
The prediction of axillary lymph node status after neoadjuvant chemotherapy (NAC) becoming critical because of the advocation of the de-escalation of axillary management. We investigate associated factors of axillary upstaging in clinical node-negative (cN0) breast cancer patients receiving NAC to develop and validate an accurate prediction nomogram.
Methods
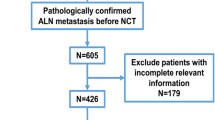
We retrospectively analyzed 1892 breast cancer patients with stage of cT1-3N0 treated by NAC and subsequent surgery between 2010 and 2020 in twenty hospitals across China. Patients randomly divided into a training set and validation set (3:1). Univariate and multivariate logistic regression analysis were performed, after which a nomogram was constructed and validated.
Results
In total, pathologic node negativity (ypN0) achieved in 1406 (74.3%) patients and another 486 (25.7%) patients upstaged to pathologic node positive (ypN+). Breast pathologic complete response (bpCR) was achieved in 445 (23.5%) patients and non-bpCR in 1447 (76.5%) patients. A nomogram was established by ER, tumor histology, HER2 status, cycle of NAC treatment, and the bpCR, which were confirmed by multivariate logistic analysis as independent predictors of nodal upstaging in the training cohort (n = 1419). The area under the receiver operating characteristic curve (AUC) of the training cohort and validation cohort (n = 473) were 0.73 (95% CI 0.693–0.751) and 0.77 (95% CI 0.723–0.812) respectively.
Conclusion
We present a nomogram with a nationwide large sample data which can effectively predict axillary upstaging after neoadjuvant chemotherapy to give better advice for individualized axillary lymph node management of breast cancer.




Similar content being viewed by others
Availability of data and material
All data during the study are proprietary in nature and may only be provided with restrictions (e.g. anonymized data).
References
Andersson Y et al (2018) Long-term breast cancer survival in relation to the metastatic tumor burden in axillary lymph nodes. Breast Cancer Res Treat 171(2):359–369
Asaoka M et al (2020) Neoadjuvant chemotherapy for breast cancer: past, present, and future. Breast Cancer (auckl) 14:1178223420980377
Barrio AV et al (2016) How often is treatment effect identified in axillary nodes with a pathologic complete response after neoadjuvant chemotherapy? Ann Surg Oncol 23(11):3475–3480
Barron AU et al (2018) Association of low nodal positivity rate among patients with ERBB2-positive or triple-negative breast cancer and breast pathologic complete response to neoadjuvant chemotherapy. JAMA Surg 153(12):1120–1126
Boér K et al (2021) Pathologic complete response rates after neoadjuvant pertuzumab and trastuzumab with chemotherapy in early stage HER2-positive breast cancer - increasing rates of breast conserving surgery: a real-world experience. Pathol Oncol Res 27:1609785
Chen P et al (2021) Laboratory indicators predict axillary nodal pathologic complete response after neoadjuvant therapy in breast cancer. Future Oncol 17(19):2449–2460
Corsi F et al (2021) Development of a novel nomogram-based online tool to predict axillary status after neoadjuvant chemotherapy in cN+ breast cancer: a multicentre study on 1,950 patients. Breast 60:131–137
Eisenhauer EA et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Feng K et al (2022) A review of studies on omitting surgery after neoadjuvant chemotherapy in breast cancer. Am J Cancer Res 12(8):3512–3531
Fisher CS et al (2020) The landmark series: axillary management in breast cancer. Ann Surg Oncol 27(3):724–729
Foldi J et al (2021) Optimal management for residual disease following neoadjuvant systemic therapy. Curr Treat Options Oncol 22(9):79
Gebruers N et al (2015) Incidence and time path of lymphedema in sentinel node negative breast cancer patients: a systematic review. Arch Phys Med Rehabil 96(6):1131–1139
Giuliano AE et al (2016) Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the american college of surgeons oncology group (Alliance) ACOSOG Z0011 randomized trial. Ann Surg 264(3):413–420
Goldhirsch A et al (2011) Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22(8):1736–1747
Gradishar WJ et al (2022) Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 20(6):691–722
Gu J et al (2022) Deep learning radiomics of ultrasonography for comprehensively predicting tumor and axillary lymph node status after neoadjuvant chemotherapy in breast cancer patients: a multicenter study. Cancer 129:356–366
Guo R et al (2020) A nomogram for predicting axillary pathologic complete response in hormone receptor-positive breast cancer with cytologically proven axillary lymph node metastases. Cancer 126(Suppl 16):3819–3829
Hammond JB et al (2022) Characterizing occult nodal disease within a clinically node-negative, neoadjuvant breast cancer population. Clin Breast Cancer 22(2):186–190
Haque W et al (2018) Response rates and pathologic complete response by breast cancer molecular subtype following neoadjuvant chemotherapy. Breast Cancer Res Treat 170(3):559–567
Hersh EH, King TA (2022) De-escalating axillary surgery in early-stage breast cancer. Breast 62(Suppl 1):S43-s49
Hwang HW et al (2019) A nomogram to predict pathologic complete response (pCR) and the value of tumor-infiltrating lymphocytes (TILs) for prediction of response to neoadjuvant chemotherapy (NAC) in breast cancer patients. Breast Cancer Res Treat 173(2):255–266
Jin X et al (2016) A nomogram for predicting the pathological response of axillary lymph node metastasis in breast cancer patients. Sci Rep 6:32585
Kang YJ et al (2022) A nomogram for predicting three or more axillary lymph node involvement before breast cancer surgery. Sci Rep 12(1):12141
Kong X et al (2022) Advances in imaging in evaluating the efficacy of neoadjuvant chemotherapy for breast cancer. Front Oncol 12:816297
Krag DN et al (2007) Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol 8(10):881–888
Lopez-Tarruella S et al (2022) How we treat HR-positive, HER2-negative early breast cancer. Future Oncol 18(8):1003–1022
Luo Y et al (2023) Axillary Downstaging and the impact of clinical axillary status on efficacy of neoadjuvant therapy for HER2-positive breast cancer: a network meta-analysis. Technol Cancer Res Treat 22:15330338221150324
Montagna G et al (2020) Selecting node-positive patients for axillary downstaging with neoadjuvant chemotherapy. Ann Surg Oncol 27(11):4515–4522
Montemurro F, Nuzzolese I, Ponzone R (2020) Neoadjuvant or adjuvant chemotherapy in early breast cancer? Expert Opin Pharmacother 21(9):1071–1082
Moorman AM, Rutgers EJT, Kouwenhoven EA (2022) Omitting SLNB in breast cancer: is a nomogram the answer? Ann Surg Oncol 29(4):2210–2218
Pilewskie M, Morrow M (2017) Axillary nodal management following neoadjuvant chemotherapy: a review. JAMA Oncol 3(4):549–555
Pilewskie M et al (2022) How often do sentinel lymph node biopsy results affect adjuvant therapy decisions among postmenopausal women with early-stage HR(+)/HER2(-) breast cancer in the post-RxPONDER era? Ann Surg Oncol 29(10):6267–6273
Reimer T et al (2020) Avoiding axillary sentinel lymph node biopsy after neoadjuvant systemic therapy in breast cancer: rationale for the prospective, multicentric EUBREAST-01 trial. Cancers (basel) 12(12):3698
Samiei S et al (2020) Correlation between pathologic complete response in the breast and absence of axillary lymph node metastases after neoadjuvant systemic therapy. Ann Surg 271(3):574–580
Samiei S et al (2021) Axillary pathologic complete response after neoadjuvant systemic therapy by breast cancer subtype in patients with initially clinically node-positive disease: a systematic review and meta-analysis. JAMA Surg 156(6):e210891
Tadros AB et al (2017) Identification of patients with documented pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery. JAMA Surg 152(7):665–670
Verbelen H et al (2019) Long-term morbidity after a negative sentinel node in breast cancer patients. Eur J Cancer Care (engl) 28(5):e13077
Weiss A et al (2021) Factors associated with nodal pathologic complete response among breast cancer patients treated with neoadjuvant chemotherapy: results of CALGB 40601 (HER2+) and 40603 (triple-negative) (alliance). Ann Surg Oncol 28(11):5960–5971
Yu CC et al (2022) Factors associated with axillary lymph node status in clinically node-negative breast cancer patients undergoing neoadjuvant chemotherapy. Cancers (Basel) 14(18):4451
Acknowledgements
We would like to thank the member units of CSBrS for data collection and the Institutional Foundation of The First Affiliated Hospital of Xi’an Jiaotong University (2022YQPY08) for supporting this work.
Funding
This work is supported by the Institutional Foundation of The First Affiliated Hospital of Xi’an Jiaotong University (2022YQPY08) for supporting this work.
Author information
Authors and Affiliations
Contributions
Conceptualization: HZ, JH, ZF and AM. Data curation: HC, PX, ZL, RL, YZ, HY, YL, KL, JZ, DM, ZY, YL, PF, JW, HJ, ZZ, XT, ZC, KW, AS, FJ, JH and ZF. Methodology: AM. Formal analysis: AM. Funding acquisition: HZ. Investigation: AM, HC, PX. Writing-original draft preparation: AM. Writing-review and editing: HZ, JH, ZF and HC. Final approval of manuscript: all authors. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that no competing interest existed.
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki and was approved by the Ethical Review Committee of the First Hospital of Jilin University (No. 2021-066). Since this study was retrospective and all data analysis was conducted anonymously, there was no informed consent of patients.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maimaitiaili, A., Chen, H., Xie, P. et al. Nomogram for predicting axillary upstaging in clinical node-negative breast cancer patients receiving neoadjuvant chemotherapy. J Cancer Res Clin Oncol 149, 8769–8778 (2023). https://doi.org/10.1007/s00432-023-04817-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-04817-9