Abstract
Purpose
To evaluate the safety and effectiveness of aflibercept in combination with fluorouracil, leucovorin, and irinotecan (FOLFIRI) in Korean patients with metastatic colorectal cancer (mCRC) who progressed with oxaliplatin-containing regimen.
Methods
This was a prospective observational study conducted at 22 sites across Korea between February 2018 and September 2019. Patients aged > 19 years with a diagnosis of mCRC who were prescribed aflibercept plus FOLFIRI, after progression with an oxaliplatin-containing regimen were included. Disease assessment was performed every 6 weeks.
Results
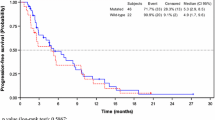
A total of 185 patients were included (males, 58.9%; right-sided tumors, 23.8%; and ECOG performance factor ≥ 1, 68.6%). A total of 514 adverse events (AEs) occurred in 134 patients, of which 206 (49.2%; 95% CI 42.0%, 56.4%) events were considered as adverse drug reactions (ADRs), 172 unexpected AEs (49.7%; 95% CI 42.5%, 56.9%), and 53 serious AEs (22.2%; 95% CI16.2%, 28.2%). The most common serious ADR was pneumonia (n = 2, 1.6%). The most common all grade hematological AE and non-hematological AE were neutropenia (21.6%) and nausea (16.2%), respectively. Over a median follow-up of 5.6 months, a total of five grade 5 (1.0%) AEs were reported. Median OS was 9.4 months, and median progression-free survival (PFS) was 7.3 months. The overall response rate was 14.6%. Right-sided tumor location and prior bevacizumab treatment were independent factors of poor PFS in multivariate analysis.
Conclusion
Aflibercept in combination with FOLFIRI was effective and showed an acceptable safety profile in Korean patients with mCRC in daily clinical practice.




Similar content being viewed by others
Availability of data material
Qualified researchers may request access to patient-level data and related documents [including, e.g., the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications]. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of study participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.
References
Adam R, Yi B, Innominato PF, Barroso E, Laurent C, Giuliante F, Capussotti L, Lapointe R, Regimbeau JM, Lopez-Ben S, Isoniemi H, Hubert C, Lin JK, Gruenberger T, Elias D, Skipenko OG, Guglielmi A, LiverMetSurvey International Contributing Centers (2017) Resection of colorectal liver metastases after second-line chemotherapy: is it worthwhile? A LiverMetSurvey analysis of 6415 patients. Eur J Cancer 78:7–15. https://doi.org/10.1016/j.ejca.2017.03.009
Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H, Colon/Rectum Carcinomas (Primary Tumor) Study Group (2010) Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum 53:57–64. https://doi.org/10.1007/DCR.0b013e3181c703a4
Benedix F, Schmidt U, Mroczkowski P, Gastinger I, Lippert H, Kube R, Study Group "Colon/Rectum Carcinoma (Primary Tumor) (2011) Colon carcinoma–classification into right and left sided cancer or according to colonic subsite? Analysis of 29,568 patients. Eur J Surg Oncol 37:134–139. https://doi.org/10.1016/j.ejso.2010.12.004
Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C, Steffens CC, Alonso-Orduña V, Schlichting C, Reyes-Rivera I, Bendahmane B, André T, Kubicka S, ML18147 Study Investigators (2013) Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 14:29–37. https://doi.org/10.1016/S1470-2045(12)70477-1
Boeckx N, Janssens K, Van Camp G, Rasschaert M, Papadimitriou K, Peeters M, Op de Beeck K (2018) The predictive value of primary tumor location in patients with metastatic colorectal cancer: a systematic review. Crit Rev Oncol Hematol 121:1–10. https://doi.org/10.1016/j.critrevonc.2017.11.003
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Bylsma LC, Gillezeau C, Garawin TA, Kelsh MA, Fryzek JP, Sangaré L, Lowe KA (2020) Prevalence of RAS and BRAF mutations in metastatic colorectal cancer patients by tumor sidedness: a systematic review and meta-analysis. Cancer Med 9:1044–1057. https://doi.org/10.1002/cam4.2747
Chau I, Fakih M, García-Alfonso P, Linke Z, Ruiz Casado A, Marques EP, Picard P, Celanovic M, Cartwright T (2020) Safety and effectiveness of aflibercept + fluorouracil, leucovorin, and irinotecan (FOLFIRI) for the treatment of patients with metastatic colorectal cancer (mCRC) in current clinical practice: OZONE study. Cancers (basel) 12:657. https://doi.org/10.3390/cancers12030657
Chong DQ, Manalo M, Imperial M, Teo P, Yong G, Ng M, Tan IB, Choo SP, Chua C (2016) Safety and efficacy of aflibercept in combination with fluorouracil, leucovorin and irinotecan in the treatment of Asian patients with metastatic colorectal cancer. Asia Pac J Clin Oncol 12:275–283. https://doi.org/10.1111/ajco.12496
Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M, Zaniboni A, Tonini G, Carlomagno C, Allegrini G, Chiara S, D’Amico M, Granetto C, Cazzaniga M, Boni L, Fontanini G, Falcone A (2015) FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 16:1306–1315. https://doi.org/10.1016/S1470-2045(15)00122-9
CTCAE (Common Terminology Criteria for Adverse Events) (2021) https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed 23 Apr 2021
Fernández Montes A, Martínez Lago N, Covela Rúa M, de la Cámara GJ, González Villaroel P, Méndez Méndez JC, Jorge Fernández M, Salgado Fernández M, Reboredo López M, Quintero Aldana G, Luz Pellón Augusto M, Graña Suárez B, García Gómez J (2019) Efficacy and safety of FOLFIRI/aflibercept in second-line treatment of metastatic colorectal cancer in a real-world population: prognostic and predictive markers. Cancer Med 8:882–889. https://doi.org/10.1002/cam4.1903
Folprecht G, Pericay C, Saunders MP, Thomas A, Lopez Lopez R, Roh JK, Chistyakov V, Höhler T, Kim JS, Hofheinz RD, Ackland SP, Swinson D, Kopp M, Udovitsa D, Hall M, Iveson T, Vogel A, Zalcberg JR (2016) Oxaliplatin and 5-FU/folinic acid (modified FOLFOX6) with or without aflibercept in first-line treatment of patients with metastatic colorectal cancer: the AFFIRM study. Ann Oncol 27:1273–1279. https://doi.org/10.1093/annonc/mdw176
Grenade C, Phelps MA, Villalona-Calero MA (2014) Race and ethnicity in cancer therapy: what have we learned? Clin Pharmacol Ther 95:403–412. https://doi.org/10.1038/clpt.2014.5
Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Lerchenmüller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Müller S, Link H, Niederle N, Rost A, Höffkes HG, Moehler M, Lindig RU, Modest DP, Rossius L, Kirchner T, Jung A, Stintzing S (2014) FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 15:1065–1075. https://doi.org/10.1016/S1470-2045(14)70330-4
Hong S, Won YJ, Park YR, Jung KW, Kong HJ, Lee ES, Community of Population-Based Regional Cancer Registries (2020) Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat 52:335–350. https://doi.org/10.4143/crt.2020.206
Ivanova JI, Saverno KR, Sung J, Duh MS, Zhao C, Cai S, Vekeman F, Peevyhouse A, Dhawan R, Fuchs CS (2017) Real-world treatment patterns and effectiveness among patients with metastatic colorectal cancer treated with ziv-aflibercept in community oncology practices in the USA. Med Oncol 34:193. https://doi.org/10.1007/s12032-017-1049-4
Kamba T, McDonald DM (2007) Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer 96:1788–1795. https://doi.org/10.1038/sj.bjc.6603813
Lavacchi D, Roviello G, Giommoni E, Dreoni L, Derio S, Brugia M, Amedei A, Pillozzi S, Antonuzzo L (2021) Aflibercept Plus FOLFIRI as second-line treatment for metastatic colorectal cancer: a single-institution real-life experience. Cancers (basel) 13:3863. https://doi.org/10.3390/cancers13153863
NCCN clinical practice guidelines in oncology (NCCN guidelines) (2021) Colon cancer version 2. 2021. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf, Accessed 08 Mar 2021
Osterlund P, Salminen T, Soveri L, Kallio R, Kellokumpu I, Lamminmäki A, Halonen P, Ristamaki R, Lantto E, Uutela A, Osterlund E, Ovissi A, Nordin A, Heerva E, Lehtomaki K, Rasanen J, Murashev M, Aroviita L, Jekunen A, Lindvall-Andersson R, Nyandoto P, Kononen J, Lepisto A, Poussa T, Muhonen T, Algars A, Isoniemi H, members of The RAXO study group (2021) Repeated centralized multidisciplinary team assessment of resectability, clinical behavior, and outcomes in 1086 Finnish metastatic colorectal cancer patients (RAXO): A nationwide prospective intervention study. Lancet Reg Health-Eur 3:100049. https://doi.org/10.1016/j.lanepe.2021.100049
Ottaiano A, Capozzi M, Tafuto S, De Stefano A, De Divitiis C, Romano C, Avallone A, Nasti G (2019) Folfiri-Aflibercept vs. Folfiri-bevacizumab as second line treatment of RAS mutated metastatic colorectal cancer in real practice. Front Oncol 9:766. https://doi.org/10.3389/fonc.2019.00766
Pastorino A, Di Bartolomeo M, Maiello E, Iaffaioli V, Ciuffreda L, Fasola G, Di Costanzo F, Frassineti GL, Marchetti P, Antoniotti C, Leone F, Zaniboni A, Aprile G, Zilocchi C, Sobrero A, Bordonaro R (2018) Aflibercept plus FOLFIRI in the real-life setting: safety and quality of life data from the Italian patient cohort of the aflibercept safety and quality-of-life program study. Clin Colorectal Cancer 17:e457–e470. https://doi.org/10.1016/j.clcc.2018.03.002
Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P, Passalacqua R, Sgroi G, Barni S (2017) Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol 3:211–219. https://doi.org/10.1001/jamaoncol.2016.4227
Price TJ, Beeke C, Ullah S, Padbury R, Maddern G, Roder D, Townsend AR, Moore J, Roy A, Tomita Y, Karapetis C (2015) Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer 121:830–835. https://doi.org/10.1002/cncr.29129
Riechelmann RP, Srimuninnimit V, Bordonaro R, Kavan P, Di Bartolomeo M, Maiello E, Cicin I, García-Alfonso P, Chau I, Fedyanin MY, Martos CF, Ter-Ovanesov M, Peeters M, Ko YJ, Yalcin S, Karthaus M, Aparicio J, Heinemann V, Picard P, Bury D, Drea E, Sobrero A (2019) Aflibercept plus FOLFIRI for second-line treatment of metastatic colorectal cancer: observations from the global aflibercept safety and health-related quality-of-life program (ASQoP). Clin Colorectal Cancer 18:183–91.e3. https://doi.org/10.1016/j.clcc.2019.05.003
Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, Nagtegaal ID, Beets-Tan RG, Arnold D, Ciardiello F, Hoff P, Kerr D, Köhne CH, Labianca R, Price T, Scheithauer W, Sobrero A, Tabernero J, Aderka D, Barroso S, Bodoky G, Douillard JY, El Ghazaly H, Gallardo J, Garin A, Glynne-Jones R, Jordan K, Meshcheryakov A, Papamichail D, Pfeiffer P, Souglakos I, Turhal S, Cervantes A (2012) ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol 23:2479–2516. https://doi.org/10.1093/annonc/mds236
Schölch S, Bogner A, Bork U, Rahbari M, Győrffy B, Schneider M, Reissfelder C, Weitz J, Rahbari NN (2019) Serum PlGF and EGF are independent prognostic markers in non-metastatic colorectal cancer. Sci Rep 9:10921. https://doi.org/10.1038/s41598-019-47429-5
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Tabernero J, Van Cutsem E, Lakomý R, Prausová J, Ruff P, van Hazel GA, Moiseyenko VM, Ferry DR, McKendrick JJ, Soussan-Lazard K, Chevalier S, Allegra CJ (2014) Aflibercept versus placebo in combination with fluorouracil, leucovorin and irinotecan in the treatment of previously treated metastatic colorectal cancer: prespecified subgroup analyses from the VELOUR trial. Eur J Cancer 50:320–331. https://doi.org/10.1016/j.ejca.2013.09.013
Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D, McKendrick J, Polikoff J, Tellier A, Castan R, Allegra C (2012) Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin based regimen. J Clin Oncol 30:3499–3506. https://doi.org/10.1200/JCO.2012.42.8201
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D’Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27:1386–1422. https://doi.org/10.1093/annonc/mdw235
Van Cutsem E, Paccard C, Chiron M, Tabernero J (2020) Impact of prior bevacizumab treatment on VEGF-A and PlGF levels and outcome following second-line aflibercept treatment: biomarker post hoc analysis of the VELOUR trial. Clin Cancer Res 26:717–725. https://doi.org/10.1158/1078-0432.CCR-19-1985
Venook AP, Niedzwiecki D, Lenz H-J, Innocenti F, Mahoney MR, O’Neil BH, Shaw JE, Polite BN, Hochster HS, Atkins JN, Goldberg RM, Mayer RJ, Schilsky RL, Bertagnolli MM, Blanke CD (2014) CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol. https://doi.org/10.1200/jco.2014.32.15_suppl.lba3
Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, Kim TW, Ismail F, Tan IB, Yeh KH, Grothey A, Zhang S, Ahn JB, Mastura MY, Chong D, Chen LT, Kopetz S, Eguchi-Nakajima T, Ebi H, Ohtsu A, Cervantes A, Muro K, Tabernero J, Minami H, Ciardiello F, Douillard JY (2018) Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS SSO and TOS. Ann Oncol 29:44–70. https://doi.org/10.1093/annonc/mdx738
Acknowledgements
The authors would like to thank the study participants, their families and caregivers who were involved in this study. Authors also thank Pravin Bolshete and Sapna Patil of Sqarona Medical Communications LLP, Mumbai for medical writing assistance which was paid for by Sanofi. Editorial support was also provided by Anahita Gouri and Rohan Mitra from Sanofi India.
Funding
The study was funded by Sanofi-Aventis Korea Co, Ltd.
Author information
Authors and Affiliations
Contributions
Conceived and designed the analysis: S-HB, JGK, JAY, DHK. Collected the data: S-HB, JGK, SHB, SHS, IP, YSP, M-AL, SL, S-YJ, S-WH, MHK, JO, JSK, JYK, MSA, DYZ, B-NB, HJJ, HKK, J-HK. Contributed data or analysis tools: JGK, S-HB, JAY, DHK. Performed the analysis: JGK, S-HB, JAY, DHK. Wrote the paper: S-HB, JGK. Supervision and revision of the manuscript: S-HB, JGK, SHB, SHS, IP, YSP, M-AL, SL, S-YJ, S-WH, MHK, JO, JSK, JYK, MSA, DYZ, B-NB, HJJ, HKK, J-HK, JAY, DHK.
Corresponding author
Ethics declarations
Conflict of interest
Ji Ae Yoon and Dong Han Kim are employees of Sanofi, and Ji Ae Yoon holds stock option. All other authors have no conflict of interest to declare.
Ethical approval
The study protocol was approved by an independent ethics committee or institutional review board at each study site. The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practices, applicable local regulatory requirements and principles that have their origin in the Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from each participant before any study-related procedure.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Beom, SH., Kim, J.G., Baik, S.H. et al. Safety and effectiveness of aflibercept in combination with FOLFIRI in Korean patients with metastatic colorectal cancer who received oxaliplatin-containing regimen. J Cancer Res Clin Oncol 149, 1131–1143 (2023). https://doi.org/10.1007/s00432-022-03946-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-03946-x