Abstract
Purpose
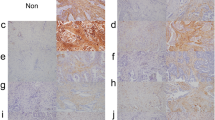
Cancer cells and cancer-associated fibroblasts (CAFs) together create the tumor microenvironment, which affects malignant behavior. Lung adenocarcinomas with CAFs expressing podoplanin (PDPN) are clinically aggressive, but the molecular mechanism underlying this phenomenon has not been established. So we identified the characteristic immunophenotype of lung adenocarcinoma cells coexisting with PDPN-expressing CAFs (PDPN-CAFs) and examined how it relates to an aggressive clinicopathological outcome.
Methods
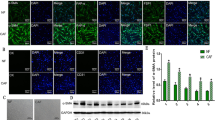
We analyzed the clinicopathological characteristics of 119 adenocarcinomas with a uniform size (2–3 cm). The expression levels of ten invasiveness-related proteins which related to cell adhesion and invasiveness, such as Ezrin, were examined in cancer cells from PDPN-CAFs (+) cases and from PDPN-CAFs (−) cases (n = 20 each). To examine the functional importance of the identified protein on the invasion phenotype, we performed wound healing and a Matrigel invasion assay using shRNA-knockdown lung adenocarcinoma cells (PC-9).
Results
The PDPN-CAFs (+) cases had significantly higher rates of node metastasis (p < 0.01) and vascular invasion (p < 0.01). The cancer cells from the PDPN-CAFs (+) cases also had a significantly higher staining score for Ezrin (p < 0.01) than those from the PDPN-CAFs (−) cases. The migration and invasion activities of the shEzrin-induced PC-9 cells were significantly lower than those of the control cells.
Conclusions
Our results indicated that within a tumor microenvironment composed of PDPN-CAFs, increased Ezrin expression in cancer cells might play a key role in the invasiveness of lung adenocarcinoma.




Similar content being viewed by others
References
Asamura H et al (2008) A Japanese Lung Cancer Registry study: prognosis of 13,010 resected lung cancers. J Thorac Oncol 3:46–52
Bretscher A, Edwards K, Fehon RG (2002) ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3:586–599
Chen QY, Xu W, Jiao DM, Wu LJ, Song J, Yan J, Shi JG (2013) Silence of ezrin modifies migration and actin cytoskeleton rearrangements and enhances chemosensitivity of lung cancer cells in vitro. Mol Cell Biochem 377:207–218
Cui Y, Li T, Zhang D, Han J (2010) Expression of Ezrin and phosphorylated Ezrin (pEzrin) in pancreatic ductal adenocarcinoma. Cancer Invest 28:242–247
Devesa SS, Bray F, Vizcaino AP, Parkin DM (2005) International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer 117:294–299
Di Cristofano C et al (2010) Phosphorylated ezrin is located in the nucleus of the osteosarcoma cell. Mod Pathol 23:1012–1020
Fehon RG, McClatchey AI, Bretscher A (2010) Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol 11:276–287
Gottschling S et al (2013) Mesenchymal stem cells in non-small cell lung cancer—different from others? Insights from comparative molecular and functional analyses. Lung Cancer 80:19–29
Hasegawa Y, Takanashi S, Kanehira Y, Tsushima T, Imai T, Okumura K (2001) Transforming growth factor-beta1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer 91:964–971
Hirao M et al (1996) Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J Cell Biol 135:37–51
Hofheinz RD et al (2003) Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie 26:44–48
Hoshino A et al (2011) Podoplanin-positive fibroblasts enhance lung adenocarcinoma tumor formation: podoplanin in fibroblast functions for tumor progression. Cancer Res 71:4769–4779
Ishii G et al (2005) In vivo and in vitro characterization of human fibroblasts recruited selectively into human cancer stroma. Int J Cancer 117:212–220
Ito TK, Ishii G, Chiba H, Ochiai A (2007) The VEGF angiogenic switch of fibroblasts is regulated by MMP-7 from cancer cells. Oncogene 26:7194–7203
Ito M, Ishii G, Nagai K, Maeda R, Nakano Y, Ochiai A (2012a) Prognostic impact of cancer-associated stromal cells in patients with stage I lung adenocarcinoma. Chest 142:151–158
Ito S et al (2012b) Tumor promoting effect of podoplanin-positive fibroblasts is mediated by enhanced RhoA activity. Biochem Biophys Res Commun 422:194–199
Karmakar S, Das C (2004) Modulation of ezrin and E-cadherin expression by IL-1beta and TGF-beta1 in human trophoblasts. J Reprod Immunol 64:9–29
Kawase A et al (2008) Podoplanin expression by cancer associated fibroblasts predicts poor prognosis of lung adenocarcinoma. Int J Cancer 123:1053–1059
Korc M (2007) Pancreatic cancer-associated stroma production. Am J Surg 194:S84–S86
Kraman M et al (2010) Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 330:827–830
Li Q et al (2012) Expression of ezrin correlates with malignant phenotype of lung cancer, and in vitro knockdown of ezrin reverses the aggressive biological behavior of lung cancer cells. Tumour Biol 33:1493–1504
Louvet-Vallee S (2000) ERM proteins: from cellular architecture to cell signaling. Biol Cell 92:305–316
Ma L, Liu YP, Zhang XH, Geng CZ, Li ZH (2013) Relationship of RhoA signaling activity with ezrin expression and its significance in the prognosis for breast cancer patients. Chin Med J (Engl) 126:242–247
Maeda M, Matsui T, Imamura M, Tsukita S, Tsukita S (1999) Expression level, subcellular distribution and rho-GDI binding affinity of merlin in comparison with Ezrin/Radixin/Moesin proteins. Oncogene 18:4788–4797
McClatchey AI (2003) Merlin and ERM proteins: unappreciated roles in cancer development? Nat Rev Cancer 3:877–883
Ohta M et al (2013) Positive and negative regulation of podoplanin expression by TGF-beta and histone deacetylase inhibitors in oral and pharyngeal squamous cell carcinoma cell lines. Oral Oncol 49:20–26
Okusa Y, Ichikura T, Mochizuki H (1999) Prognostic impact of stromal cell-derived urokinase-type plasminogen activator in gastric carcinoma. Cancer 85:1033–1038
Pujuguet P, Del Maestro L, Gautreau A, Louvard D, Arpin M (2003) Ezrin regulates E-cadherin-dependent adherens junction assembly through Rac1 activation. Mol Biol Cell 14:2181–2191
Scott AM et al (2003) A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin Cancer Res 9:1639–1647
Suzuki H, Kato Y, Kaneko MK, Okita Y, Narimatsu H, Kato M (2008) Induction of podoplanin by transforming growth factor-beta in human fibrosarcoma. FEBS Lett 582:341–345
Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, Takai Y (1997) Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem 272:23371–23375
Tsukita S, Oishi K, Sato N, Sagara J, Kawai A, Tsukita S (1994) ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol 126:391–401
Tynninen O, Carpen O, Jaaskelainen J, Paavonen T, Paetau A (2004) Ezrin expression in tissue microarray of primary and recurrent gliomas. Neuropathol Appl Neurobiol 30:472–477
Xing F, Saidou J, Watabe K (2010) Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci 15:166–179
Xu Y, Yu Q (2003) E-cadherin negatively regulates CD44-hyaluronan interaction and CD44-mediated tumor invasion and branching morphogenesis. J Biol Chem 278:8661–8668
Yoshizawa A et al (2011) Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 24:653–664
Zhang XQ, Chen GP, Wu T, Yan JP, Zhou JY (2012) Expression and clinical significance of ezrin in non-small-cell lung cancer. Clin Lung Cancer 13:196–204
Acknowledgments
This work was supported by National Cancer Center Research and Development Fund (23-A-12 and 23-K-18), the Foundation for the Promotion of Cancer Research, 3rd-Term Comprehensive 10-Year Strategy for Cancer Control, the Advanced research for medical products Mining Programme of the National Institute of Biomedical Innovation (NIBIO), and JSPS KAKENHI (24659185).
Conflict of interest
The authors have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
432_2014_1851_MOESM1_ESM.pdf
Supplementary Figure 1 Western blot analysis showing Ezrin expression in control (shLuciferase) or Ezrin shRNA-induced PC-9 cells, compared with β-actin expression (PDF 71 kb)
Rights and permissions
About this article
Cite this article
Suzuki, S., Ishii, G., Matsuwaki, R. et al. Ezrin-expressing lung adenocarcinoma cells and podoplanin-positive fibroblasts form a malignant microenvironment. J Cancer Res Clin Oncol 141, 475–484 (2015). https://doi.org/10.1007/s00432-014-1851-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1851-8