Abstract
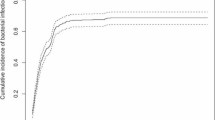
Patients with high-risk neuroblastoma (HR-NB) exhibit suboptimal 5-year survival rates, leading to a widespread international preference for high-intensity chemotherapeutic regimens in these children. We analyzed the incidence and risk factors for complications during induction chemotherapy in children with HR-NB and tried to assist clinicians in predicting such complications and optimizing therapeutic strategy. The clinical data of children with HR-NB admitted to our hospital from January 2007 to December 2019 were retrospectively analyzed. The incidence, characteristics, and risk factors of complications (infection, hemorrhage, and chemotherapy-related adverse reactions (CRAR)) requiring hospitalization during induction chemotherapy in these children were explored. (1) A total of 108 patients with HR-NB were included in the final analysis. The overall infection rate was 92.6% (100/108), with the highest incidence of 71.3% observed during the first cycle. FN, bacterial infection, as well as fungal infection were common infectious complications in children with HR-NB during induction chemotherapy. (2) The overall hemorrhage rate was 24.1% (26/108), with the highest incidence of 14.8% also observed in the first cycle. Among the children with hemorrhage, there were 72% with bone marrow involved, while 65.0% of them had a high vanillylmandelic acid (VMA) value. And children with hemorrhage also exhibited neuron-specific enolase (NSE) ≥ 200 µg/L in 88.5% of cases and lactic dehydrogenase (LDH) ≥ 1000U/L in 73.1% of cases. (3) The incidence of CRAR rate was 100%, and 99.1% (107/108) patients experienced myelosuppression. The incidence of myelosuppression peaked in the third cycle, reaching up to 85.2%. Most children suffered severe myelosuppression existed with bone marrow metastases (76.3%), abnormal VMA (67.5%), and LDH ≥ 1000 U/L (60%). (4) Non-myelosuppressive adverse effects were observed in 75.9% children (82/108), with the highest incidence occurring in the third cycle at 42.6%. (5) Patients who experienced three types of complications had a lower median survival time (MST) of 54.4 months, a 3-year event-free survival (EFS) rate of (44.2 ± 10.7)%, and a 3-year overall survival (OS) rate of (75.8 ± 8.6)%, in comparison to those with only one or two complications, who had a higher MST of 59.5 months, a 3-year EFS rate of (73.5 ± 5.2)% (X2 = 10.457, P = 0.001), and a 3-year OS rate of (84.8 ± 4.1)% (X2 = 10.511, P = 0.001).
Conclusion: The presence of bone marrow involved and increased VMA were high-risk factors for infection, while NSE ≥ 200 µg/L and LDH ≥ 1000 U/L were high-risk factors for hemorrhage. For those children who had experienced severe myelosuppression, the presence of bone marrow metastases, increased VMA, and LDH ≥ 1000 U/L were their risk factors. The presence of bone involvement was a high-risk factor for children to have non-myelosuppressive adverse effects. Complications that arise during induction chemotherapy could negatively impact the children’s prognosis and overall quality of life.
What is Known: • The high-risk neuroblastoma (HR-NB) had a worse prognosis; there was a general international preference for high-intensity chemotherapeutic regimens in the induction phase to these children. | |
What is New: • We analyzed the incidence and risk factors of complications during induction chemotherapy in children with HR-NB and tried to help clinicians predict such complications and adopt optimized therapeutic strategy. |











Similar content being viewed by others
Data availability
The data associated with our research are available upon request from the corresponding author. We are more than willing to provide the necessary data to interested researchers upon reasonable request.
Abbreviations
- CCCG:
-
Chinese Children’s Cancer Group
- CDDP:
-
Cisplatin
- COG:
-
Children’s Oncology Group
- CRAR:
-
Chemotherapy-related adverse reactions
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- CTX:
-
Cyclophosphamide
- DOXO:
-
Doxorubicin
- FN:
-
Febrile neutropenia
- G-CSF:
-
Granulocyte colony-stimulating factors
- HR-NB:
-
High-risk neuroblastoma
- INPC:
-
The International Neuroblastoma Pathology Classification
- INSS:
-
International Neuroblastoma Staging System
- LDH:
-
Lactic dehydrogenase
- MST:
-
Median survival time
- NB:
-
Neuroblastoma
- NSE:
-
Neuron-specific enolase
- EFS:
-
Event-free survival
- OS:
-
Overall Survival
- PBSC:
-
Peripheral blood stem cell
- PICU:
-
Pediatric Intensive Care Unit
- sAML:
-
Secondary acute myeloid leukemia (sAML)
- TLS:
-
Tumor lysis
- TOPO:
-
Topotecan
- VCR:
-
Vincristine
- VIPN:
-
Peripheral neurotoxicity
- VP-16:
-
Etoposide
References
Zhen H, Guan H, Ma J, Wang W, Jing S, Miao Z et al (2021) Risk of developing second malignant neoplasms in patients with neuroblastoma: a population study of the US SEER database. Radiat Oncol 16(1):228. https://doi.org/10.1186/s13014-021-01943-x
Maris JM (2010) Recent advances in neuroblastoma. N Engl J Med 362(23):2202–2211. https://doi.org/10.1056/NEJMra0804577
Sun Q, Chen Y, Jin Q, Yuan X (2022) A nomogram for predicting recurrence-free survival of intermediate and high-risk neuroblastoma. Eur J Pediatr. https://doi.org/10.1007/s00431-022-04617-2
von Allmen D, Davidoff AM, London WB, Van Ryn C, Haas-Kogan DA, Kreissman SG et al (2017) Impact of extent of resection on local control and survival in patients from the COG A3973 study with high-risk neuroblastoma. J Clin Oncol 35(2):208–216. https://doi.org/10.1200/JCO.2016.67.2642
Zhang D, Kaweme NM, Duan P, Dong Y, Yuan X (2021) Upfront treatment of pediatric high-risk neuroblastoma with chemotherapy, surgery, and radiotherapy combination: the CCCG-NB-2014 protocol. Front Oncol 11:745794. https://doi.org/10.3389/fonc.2021.745794
Olsen HE, Campbell K, Bagatell R, DuBois SG (2020) Trends in conditional survival and predictors of late death in neuroblastoma. Pediatr Blood Cancer 67(10):e28329. https://doi.org/10.1002/pbc.28329
Whittle SB, Williamson KC, Russell HV (2017) Incidence and risk factors of bacterial and fungal infection during induction chemotherapy for high-risk neuroblastoma. Pediatr Hematol Oncol 34(5):331–342. https://doi.org/10.1080/08880018.2017.1396386
Ward E, DeSantis C, Robbins A, Kohler B, Jemal A (2014) Childhood and adolescent cancer statistics. CA Cancer J Clin 64(2):83–103. https://doi.org/10.3322/caac.21219
Zhu J, Wang J, Sun F, Zhen Z, Chen T, Lu S et al (2022) Vincristine, irinotecan, and temozolomide in patients with relapsed/refractory neuroblastoma. Front Oncol 12:804310. https://doi.org/10.3389/fonc.2022.804310
Voglino V, Persano G, Crocoli A, Castellano A, Serra A, Giordano U et al (2021) Hemorrhage during induction chemotherapy in neuroblastoma: additional risk factors in high-risk patients. Front Pediatr 9:761896. https://doi.org/10.3389/fped.2021.761896
Triarico S, Romano A, Attina G, Capozza MA, Maurizi P, Mastrangelo S et al (2021) Vincristine-induced peripheral neuropathy (VIPN) in Pediatric tumors: mechanisms, risk factors, strategies of prevention and treatment. Int J Mol Sci 22(8). https://doi.org/10.3390/ijms22084112
McNerney ME, Godley LA, Le Beau MM (2017) Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer 17(9):513–527. https://doi.org/10.1038/nrc.2017.60
Rogers AE, Eisenman KM, Dolan SA, Belderson KM, Zauche JR, Tong S et al (2017) Risk factors for bacteremia and central line-associated blood stream infections in children with acute myelogenous leukemia: a single-institution report. Pediatr Blood Cancer 64(3). https://doi.org/10.1002/pbc.26254
Sung L, Aplenc R, Alonzo TA, Gerbing RB, Lehrnbecher T, Gamis AS (2013) Effectiveness of supportive care measures to reduce infections in pediatric AML: a report from the Children’s oncology group. Blood 121(18):3573–3577. https://doi.org/10.1182/blood-2013-01-476614
Masse IO, Guillemette S, Laramee ME, Bronchti G, Boire D (2014) Strain differences of the effect of enucleation and anophthalmia on the size and growth of sensory cortices in mice. Brain Res 1588:113–126. https://doi.org/10.1016/j.brainres.2014.09.025
Inaba H, Pei D, Wolf J, Howard SC, Hayden RT, Go M et al (2017) Infection-related complications during treatment for childhood acute lymphoblastic leukemia. Ann Oncol 28(2):386–392. https://doi.org/10.1093/annonc/mdw557
Committee CA-CAPTS, Group CMAPSBO (2022) Expert consensus CCCG-NB-2021 program for the treatment of childhood neuroblastoma. Chin J Pediatr Surg 43(07):588–598. https://doi.org/10.3760/cma.j.cn421158-20211227-00638
Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM et al (2009) The International Neuroblastoma Risk Group (INRG) classification system: an INRG task force report. J Clin Oncol 27(2):289–297. https://doi.org/10.1200/JCO.2008.16.6785
Shimada H, Ambros IM, Dehner LP, Hata JI, Joshi VV, Roald B et al (1999) The international neuroblastoma pathology classification (the Shimada system). Cancer: Interdiscip Int J Am Cancer Soc 86(2):364–372. https://acsjournals.onlinelibrary.wiley.com/doi/full/10.1002/%28SICI%291097-0142%2819990715%2986%3A2%3C364%3A%3AAID-CNCR21%3E3.0.CO%3B2-7
Afzal S, Ethier M-C, Dupuis LL, Tang L, Punnett AS, Richardson SE et al (2009) Risk factors for infection-related outcomes during induction therapy for childhood acute lymphoblastic leukemia. Pediatr Infect Dis J 28(12):1064–1068. https://doi.org/10.1097/INF.0b013e3181aa6eae
O’Connor D, Bate J, Wade R, Clack R, Dhir S, Hough R et al (2014) Infection-related mortality in children with acute lymphoblastic leukemia: an analysis of infectious deaths on UKALL2003. Blood 124(7):1056–1061. https://doi.org/10.1182/blood-2014-03-560847
Rivera-Salgado D, Valverde-Muñoz K, Ávila-Agüero ML (2018) Neutropenia febril en niños con cáncer: manejo en el servicio de emergencias. Rev Chilena Infectol 35(1):62–71. https://doi.org/10.4067/s0716-10182018000100062
Ladenstein R, Valteau-Couanet D, Brock P, Yaniv I, Castel V, Laureys G et al (2010) Randomized trial of prophylactic granulocyte colony-stimulating factor during rapid COJEC induction in pediatric patients with high-risk neuroblastoma: the European HR-NBL1/SIOPEN study. J Clin Oncol 28(21):3516–3524. https://doi.org/10.1200/JCO.2009.27.3524
Qin H, Yang S, Cai S, Ren Q, Han W, Yang W et al (2020) Clinical characteristics and risk factors of 47 cases with ruptured neuroblastoma in children. BMC Cancer 20(1):243. https://doi.org/10.1186/s12885-020-06720-9
Shiokawa N, Okamoto Y, Kodama Y, Nishikawa T, Tanabe T, Mukai M et al (2016) Conservative treatment of massive hemothorax in a girl with neuroblastoma. Pediatr Int 58(10):1090–1092. https://doi.org/10.1111/ped.13094
Lode HN, Henze G, Siebert N, Ehlert K, Barthlen W (2019) Management of tumor rupture and abdominal compartment syndrome in an infant with bilateral high risk stage 4 neuroblastoma: a case report. Medicine (Baltimore) 98(34):e16752. https://doi.org/10.1097/MD.0000000000016752
Skoetz N, Haque M, Weigl A, Kuhr K, Monsef I, Becker I et al (2017) Antiemetics for adults for prevention of nausea and vomiting caused by moderately or highly emetogenic chemotherapy: a network meta-analysis. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.Cd012775
Fabi A, Malaguti P (2013) An update on palonosetron hydrochloride for the treatment of radio/chemotherapy-induced nausea and vomiting. Expert Opin Pharmacother 14(5):629–641. https://doi.org/10.1517/14656566.2013.771166
Brennan-Jones CG, McMahen C, Van Dalen EC (2019) Cochrane corner: platinum-induced hearing loss after treatment for childhood cancer. Int J Audiol 58(4):181–184. https://doi.org/10.1080/14992027.2018.1539808
Clemens E, de Vries AC, Am Zehnhoff-Dinnesen A, Tissing WJ, Loonen JJ, Pluijm SF et al (2017) Hearing loss after platinum treatment is irreversible in noncranial irradiated childhood cancer survivors. Pediatr Hematol Oncol 34(2):120–129. https://doi.org/10.1080/08880018.2017.1323985
Frisina RD, Wheeler HE, Fossa SD, Kerns SL, Fung C, Sesso HD et al (2016) Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin-based chemotherapy in survivors of adult-onset cancer. J Clin Oncol 34(23):2712–2720. https://doi.org/10.1200/JCO.2016.66.8822
Wei M, Yuan X (2019) Cisplatin-induced ototoxicity in children with solid tumor. J Pediatr Hematol Oncol 41(2). https://doi.org/10.1097/MPH.0000000000001282
Freyer DR, Chen L, Krailo MD, Knight K, Villaluna D, Bliss B et al (2017) Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol 18(1):63–74. https://doi.org/10.1016/s1470-2045(16)30625-8
Brock PR, Maibach R, Childs M, Rajput K, Roebuck D, Sullivan MJ et al (2018) Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med 378(25):2376–2385. https://doi.org/10.1056/NEJMoa1801109
Yang QY, Hu YH, Guo HL, Xia Y, Zhang Y, Fang WR et al (2021) Vincristine-induced peripheral neuropathy in childhood acute lymphoblastic leukemia: genetic variation as a potential risk factor. Front Pharmacol 12:771487. https://doi.org/10.3389/fphar.2021.771487
van de Velde ME, van den Berg MH, Kaspers GJL, Abbink FCH, Twisk JWR, van der Sluis IM et al (2021) The association between vincristine-induced peripheral neuropathy and health-related quality of life in children with cancer. Cancer Med 10(22):8172–8181. https://doi.org/10.1002/cam4.4289
Barnett S, Hellmann F, Parke E, Makin G, Tweddle DA, Osborne C et al (2022) Vincristine dosing, drug exposure and therapeutic drug monitoring in neonate and infant cancer patients. Eur J Cancer (Oxford, England : 1990) 164:127–136. https://doi.org/10.1016/j.ejca.2021.09.014
Applebaum MA, Vaksman Z, Lee SM, Hungate EA, Henderson TO, London WB et al (2017) Neuroblastoma survivors are at increased risk for second malignancies: a report from the International Neuroblastoma Risk Group Project. Eur J Cancer 72:177–185. https://doi.org/10.1016/j.ejca.2016.11.022
Kreissman SG, Seeger RC, Matthay KK, London WB, Sposto R, Grupp SA et al (2013) Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol 14(10):999–1008. https://doi.org/10.1016/s1470-2045(13)70309-7
Morton LM, Dores GM, Schonfeld SJ, Linet MS, Sigel BS, Lam CJK et al (2019) Association of chemotherapy for solid tumors with development of therapy-related myelodysplastic syndrome or acute myeloid leukemia in the modern era. JAMA Oncol 5(3):318–325. https://doi.org/10.1001/jamaoncol.2018.5625
Ma X, Liu Y, Liu Y, Alexandrov LB, Edmonson MN, Gawad C et al (2018) Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature 555(7696):371–376. https://doi.org/10.1038/nature25795
Acknowledgements
The authors wish to thank the patients, their parents, and all staff in the Department of Pediatric Hematology/Oncology, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Data collection and analysis were performed by Jiaxi Du, who also wrote the initial draft of the manuscript. Xiaojun Yuan commented on previous versions of the manuscript and made revisions.
Corresponding author
Ethics declarations
Ethics approval
The ethics approval was granted by the ethics committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine with approval number XHEC-D-2023-008. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, J., Yuan, X. Analysis of the incidence, characteristics, and risk factors of complications during induction chemotherapy in children with high-risk neuroblastoma. Eur J Pediatr 183, 185–202 (2024). https://doi.org/10.1007/s00431-023-05273-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-05273-w