Abstract
Coronavirus disease 2019 (COVID-19) infection in pediatric patients with autoimmune disorders is an area of particular concern since autoimmune diseases can increase the risk of complications from the virus. However, as the infection rates were significantly higher in adults compared to children, this at-risk group of children was relatively underrepresented in COVID-19 research. The underlying inflammatory basis of autoimmune diseases and medications that affect the immune system, such as corticosteroids, could increase the risk of severe infection in this group of patients. COVID-19 could reportedly lead to a variety of alterations in the immune system. These alterations are plausibly dependent on the underlying immune-mediated diseases or prior use of immunomodulatory drugs. Patients administrating immunomodulatory agents, especially those with severe immune system dysregulation, can experience severe symptoms of COVID-19. Nonetheless, receiving immunosuppressive medications can benefit patients by preventing cytokine storm syndromes and lung tissue damage, threatening outcomes of COVID-19.
Conclusion: In this review, we sought to evaluate the currently available literature on the impact of autoimmune disease and its related therapeutic approaches on the COVID-19 infection course of disease in children and reflect on the gaps in the evidence and the need for further research in this field.
What is Known: • The majority of children infected with COVID-19 demonstrate mild to moderate clinical manifestations compared to adults, whereas those children with pre-existing autoimmune conditions are at a greater risk for severe symptoms. •There is currently limited understanding of the pathophysiology and clinical outcomes of COVID-19 in pediatric patients with autoimmune disorders due to scattered reports and inadequate evidence. |
What is New: • Generally, children with autoimmune disorders have more unfavorable outcomes than healthy children; yet, the severity is not extreme, and is highly dependent on their autoimmune disease type and severity, as well as the medication they are taking. |
Similar content being viewed by others
Introduction
On January 7, 2020, a new coronavirus was discovered in lung fluid from a patient with pneumonia-like symptoms in Wuhan, China, using meta-transcriptomic sequencing [1]. Since then, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the pathogen that causes coronavirus disease 2019 (COVID-19), has produced extreme levels of morbidity and mortality around the world [2]. In March 2020, the World Health Organization (WHO) proclaimed the novel coronavirus outbreak a pandemic [3]. SARS-CoV-2 is an enveloped, positive-sense, single-stranded ribonucleic acid (RNA) virus belonging to the Coronaviridae family [4]. It is the seventh coronavirus to infect humans, following the discovery of SARS and Middle East respiratory syndrome viruses (MERS) [5]. The virus attacks the lower respiratory tract and causes pneumonia in humans. The symptoms are milder than those of SARS or MERS, but the disease can eventually become lethal due to hyperinflammation and respiratory failure [6].
COVID-19 has been detected in 759,408,703 people globally, with 6,866,434 deaths reported by WHO on 7 March 2023 [7]. SARS-CoV-2 cases in children under 18 are estimated to account for 2%–5% of all SARS-CoV-2 cases worldwide [8]. Albeit, it must be considered that most children with COVID-19 infection are asymptomatic or have non-specific mild to moderate symptoms. Moreover, due to the prioritization of broad COVID-19 testing for adults and patients with severe symptoms, the reported incidence of SARS-CoV-2 infection in children might be underestimated [9]. However, by June 10, 2022, over 13,400 deaths in people under the age of 20 were reported, with 58% of deaths occurring among teenagers aged 10 to 19, and 42% among those aged 0 to 9 years old [10].
Fever was the most common symptom, followed by cough, nasal symptoms, diarrhea, and nausea/vomiting in COVID-19-infected children [11]. Comorbidities, including obesity, diabetes, heart diseases, chronic lung diseases, epilepsy, and immunocompromised conditions, were identified as risk factors for higher morbidity and mortality of COVID-19 among children [12, 13].
Patients with autoimmune diseases are also reported to be at a higher risk of COVID-19 infection [14]. This could be ascribed to the regular use of glucocorticoids and other anti-rheumatoid agents in this group of patients [15]. Research also found that genetic and epigenetic factors related to autoimmune diseases like lupus, rheumatoid arthritis, type 1 diabetes, and Graves' disease might increase the risk and severity of SARS-CoV-2 infections [16,17,18]. Investigations of COVID-19 infection in individuals with autoimmune diseases and the pathologic effect of these conditions on COVID-19 severity have yet to be small in scale and generally region-specific. As a result, the effects of COVID-19 on patients with autoimmune diseases are still unknown [19,20,21]. In addition, because of the low prevalence of pediatric cases, clear conclusions about the natural history of COVID-19 in pediatric patients with autoimmune diseases have been even more difficult to obtain [8].
Accordingly, the present study was designed to comprehensively explore what is currently known and what knowledge we still lack about the pathophysiology of COVID-19, diagnosis, prognosis, clinical outcomes, severity, and treatment in children with the most common autoimmune diseases.
Type 1 diabetes
Chronic hyperglycemia caused by abnormalities in insulin secretion, insulin action, or both characterizes the group of metabolic illnesses known as diabetes mellitus [22]. An autoimmune disease known as type 1 diabetes promotes the loss of pancreatic beta cells, resulting in insufficient insulin production and hyperglycemia. Since type 1 diabetes is a chronic condition, it requires constant insulin replacement and intensive blood glucose level control by the patient [23]. Less than half of people with type 1 diabetes are diagnosed before the age of 15, even though type 1 diabetes accounts for more than 90% of children and adolescents with diabetes in most western countries [22].
Impaired glucose metabolism and immune system dysregulation
High blood sugar promotes SARS-CoV-2 replication in human monocytes, and glycolysis maintains SARS-CoV-2 replication by activating hypoxia-inducible factor 1α20 and producing reactive oxygen species in the mitochondri [24]. Therefore, viral growth may be aided by hyperglycemia. The finding supports this theory that hyperglycemia or a history of Type-1 Diabetes Mellitus (T1DM) and Type-2 Diabetes Mellitus (T2DM) are independent predictors of morbidity and mortality in SARS21 patients [25]. Poor glycemic control and higher HbA1c level are associated with inflammation, hypercoagulability, and low oxygen saturation in COVID-19 patients, predicting an increased need for medications and hospitalizations and a higher mortality rate [26, 27]. Notably, those with impaired glucose regulation or diabetes mellitus frequently have glycemic deterioration as a COVID-19 complication [28].
Dysregulated immunological condition is associated with macrovascular consequences of diabetes mellitus, as hyperglycemia can impair immune function [29, 30]. Accordingly, acute respiratory virus infection(Influenza, Human respiratory syncytial virus (HRSV)) boosts IFN production and results in muscular insulin resistance in humans. This results in compensatory hyperinsulinemia, which stabilizes blood sugar levels and stimulates antiviral CD8+ T cell responses. Such compensation might not be successful in patients with impaired glucose tolerance or diabetes mellitus. It should be noted that by promoting CD8+ effector T cell activity directly, hyperinsulinemia can boost antiviral immunity. [31, 32] Reduced NK cell activity also appears in people with impaired glucose tolerance or diabetes mellitus, which may help to explain why people with diabetes mellitus are more vulnerable to COVID-19 and have a worse prognosis than people without the condition [33].
COVID-19 outcomes in children with type 1 diabetes
There is growing evidence that children with type 1 diabetes are at a higher risk of severe COVID-19 illness (i.e., experiencing ICU admission, intermittent mandatory ventilation, or death) [12, 34]. Patients with diabetes mellitus are more likely to experience thromboembolic consequences and damage to key organs due to factors such as glucotoxicity, endothelium damage from inflammation, oxidative stress, and cytokine production [35]. In addition, antiviral medications or systemic corticosteroids, frequently used in the treatment of COVID-19 patients, may further worsen hyperglycemia [33].
It is shown that mortality rate, the relative risk of endotracheal intubation, and septic shock were increased in children with type 1 diabetes and Covid-19 than in children with Covid-19 and no Type 1 diabetes [36]. Even though well-controlled type 1 diabetes patients do not have an increased risk of infection. Special attention should be given to patients with poor glycemic control, who are at an elevated risk of infection in general due to deficient immune systems, hyperglycemia, and developing diabetic ketoacidosis (DKA). Uncontrolled diabetic children are also at a higher risk of hospitalization and infection-related complications [37,38,39,40,41,42,43,44] (Table 1). For instance, several reports have addressed COVID-19 post-inflammatory response in children with type 1 diabetes, About 2–4 weeks following the onset of COVID-19 in children; There have been cases of children acquiring the uncommon but serious condition known as a multisystem inflammatory syndrome (MIS-C). A cluster of children with hyperinflammatory shock with symptoms resembling Kawasaki illness and toxic shock syndrome was described at the height of the COVID-19 pandemic. Most MIS-C cases exhibit shock-like characteristics, including cardiac involvement, gastrointestinal symptoms, noticeably raised inflammatory markers, and positive SARS-CoV-2 laboratory test results [45,46,47,48,49].
Rheumatic diseases
One of the most prevalent chronic disorders in children is rheumatic disease. They often affect several organ systems and are complicated. Juvenile idiopathic arthritides are the most prevalent subgroup of pediatric rheumatic illnesses, followed by juvenile-onset systemic lupus erythematosus (SLE), and less common conditions such as juvenile dermatomyositis, primary vasculitides, and scleroderma [54].
Rheumatic disorders are related to a higher infection risk than the general population without the disease [55]. The primary cause is a general immune system impairment common to all autoimmune diseases, which depends on the disease activity level [56]. Studies revealed that active disease and receiving immunomodulatory drugs, i.e. corticosteroids and non-steroidal anti-inflammatory medications, are significant risk factors for an increased rate of infection [56,57,58,59,60,61,62]. Even when patients with rheumatic disorders stop receiving their established immunomodulatory and anti-inflammatory medicines, these treatments may negatively impact their immunological system. In the early phases of COVID-19, the diminished body's immune response and antiviral defense followed by immune-suppressant agents may harm the course of the infection. However, later on, it might also support and even help to avoid cytokine storm syndrome in the severe course of COVID-19 [63]. In addition, some other drugs administered in rheumatic diseases, like hydroxychloroquine, methotrexate, and tocilizumab, have not been associated with an increased risk of respiratory infections and even might have a therapeutic role in the treatment of COVID-19 [64, 65].
On the other hand, data have shown gene mutations in some rheumatic diseases that may lead to the cytokine storm syndrome associated with COVID-19 [66, 67]. Sawalha et al. reported that epigenetic dysregulations in SLE resulted in angiotensin-converting enzyme 2 expressions (ACE2) overexpression, and might cause a specific susceptibility to COVID-19 [17]. Comorbidities, which commonly exacerbate Rheumatoid Arthritis (RA), are another critical factor in determining the risk of infection [68]. Chronic obstructive pulmonary disease (COPD), interstitial lung disease, renal failure, diabetes mellitus, and cardiovascular disease are all concurrent conditions linked to an increased prevalence of infections in RA [62, 69].
COVID-19 outcomes in children with rheumatic diseases
Given all factors modifying the COVID-19 susceptibility and severity in patients with rheumatic diseases, children are more unlikely to have serious rheumatic disease course; hence, they are less likely to develop severe COVID-19 infection solely because of their underlying rheumatic disease [61, 70]. In conclusion, further studies are warranted to better clarify COVID-19 severity in children with rheumatic diseases [58,59,60,61, 70].
To date, little data are available on rheumatic diseases and COVID-19, particularly among children. However, a meta-analysis showed that immune-compromised situations such as rheumatic diseases requiring immunosuppressive treatment were not a risk factor for more severe COVID-19 disease courses. Furthermore, those with and without rheumatic and musculoskeletal diseases (RMD) had nearly the same rate of hospitalization, ICU admission, and mechanical ventilation. In contrast, the mortality rate was increased in patients with RMDs (Odds ratio(OR) 1.74 [95% Confidence interval(CI) 1.08–2.80]) [71].
Several studies showed that the clinical course of COVID-19 was mild in children with rheumatic disease, with most of them being asymptomatic, regardless of whether immunosuppressive therapy was maintained or discontinued. However, there were some other studies and case report that documented severe outcomes and complications in children [11, 58,59,60,61, 72,73,74,75,76,77,78,79] (Table 2). Medium/high-dose corticosteroids, the use of mycophenolate and rituximab, and significant immunosuppression were found to be hospitalization risk factors in a case series investigating pediatric patients with rheumatic illnesses and laboratory-confirmed COVID-19. High-risk signs for admission were fever, dyspnea, chest pain, and rash. The requirement for hospitalization may be influenced by the activity and flare of rheumatic diseases [75]. In patients with underlying risk factors for hyperinflammation, COVID-19 may cause mortality regardless of the administration of biological Disease-modifying antirheumatic drugs(bDMARD) [76].
Moreover, numerous rheumatic disorders were identified in survey-based cohort research in children, and immunosuppressive therapy does not appear to increase the risk. On the other hand, abruptly stopping these medications could result in clinical instability and aggravation of the underlying condition. Additionally, one should be cautious to avoid COVID-19-related disease exacerbations [74].
In summary, there is controversial evidence, but it appears that comorbidities, higher disease activity, and oral corticosteroids can be risk factors for severe SARS-CoV-2 infection.
Autoimmune neurological diseases
Although COVID-19 is typically thought of as a respiratory disease, numerous reports have noted that COVID-19 also has neurologic symptoms [84]. An alarming number of neurological symptoms associated with COVID-19 have been reported, ranging from minor ones like headaches and myalgia to serious ones like stroke and encephalopathy [85]. With 45.5% of those with severe COVID-19 infection experiencing neurologic symptoms compared to 30.2% of those with non-severe infection, severe infection was linked to a greater prevalence of neurologic manifestations [84]. Compared to the adult population, fewer neurologic consequences of COVID-19 have been identified in pediatric patients. In children, most symptoms are restricted to headache and/or loss of smell or taste [86]. There are, however, case reports documenting more serious neurologic issues, such as encephalitis, seizures, and cerebrovascular stroke in children [86,87,88,89,90,91,92,93,94,95].
ACE2 is expressed on the blood–brain barrier (BBB)’s vasculature and facilitates SARS transport by targeting the spike protein [96]. The olfactory bulb and the cribriform plate are direct entry points for SARS-CoV-2 into the central nervous system, where it can cause neuronal injury and prevent oligodendrocyte differentiation and remyelination. The loss of microglial cells impairs the removal of myelin debris, and the release of neurotoxic soluble substances from activated astrocytes contributes to pathological reactions. In addition, SARS-CoV2 can trigger the development of CNS-specific lymphocytes through molecular mimicry. It is crucial to note that the BBB is damaged from the inside and outside, making it easier for peripheral immune cells to infiltrate the CNS parenchyma. This is because of the neurotropic and hematogenous routes of infection and disease impingement on the BBB [97]. The potential for molecular mimicry and the stimulation of auto-reactive T cells against myelin is also worthy to note. Through this possible mechanism, SARSE-COV 2 can cause disease exacerbation or develop new other autoimmune diseases [98].
It is possible that COVID-19's invasion of CNS could make any existing neurologic damage worse [99]. This is particularly relevant in the setting of neurological disorders with an autoimmune base, like multiple sclerosis (MS), as patients with these conditions already have increased cytokine levels and are regularly taking disease-modifying treatments (DMTs) that can impair their immune systems [100]. When the BBB breaks down, immune cells infiltrate and help to promote demyelination. COVID-19 causes vascular injury and elicits a generalized inflammatory response, which may exacerbate the breakdown of the BBB and accelerate the course of multiple sclerosis (MS) [99]. The most prevalent acute CNS demyelinating illness, acute disseminated encephalomyelitis, primarily affects younger adults and children.
Acute disseminated encephalomyelitis (ADEM) is an inflammatory demyelinating disease of the central nervous system that primarily affects children under 10 years old and is more common in males. The disease typically develops 1–2 weeks after infections or, less frequently, after vaccinations. It affects multifocal areas of the white matter, rarely the gray matter, and the spinal cord [101]. In patients with a genetic predisposition, ADEM pathophysiology is hypothesized to be connected to the antigenic mimicry theorem [102]. To put it another way, a recent viral or bacterial infection stimulates the immune system, particularly the T-helper 2 (Th2) cells and neutrophils, which causes excessive production of cytokines and chemokines [103, 104]. Infection with COVID-19 has been associated with cytokine storms that harm the central nervous system [105]. Thus, COVID-19 infection could exacerbate an already ongoing ADEM illness. Nevertheless, this does not negate the possibility that COVID-19 systemic infection can induce ADEM on its own [106].
The adverse events following the immunotherapies are the primary concern with COVID-19 in all neuro-immunological diseases. One important component is the systemic, long-term alteration of the immune response brought on by corticosteroids, immunosuppressants, or DMT medications [107]. DMTs can be grouped into the following categories, albeit classification is not entirely correct, as different DMTs have diverse modes of action: 1) Interferon β-1 (IFN-β1), glatiramer acetate (GA), and fumarates (e.g., dimethyl fumarate) are immunomodulators. 2) Cell trafficking changes are caused by agents like S1P receptor modulators (e.g., fingolimod) and natalizumab, an anti-4-integrin antibody). 3) cell depletion is caused by anti-CD20 antibodies (e.g. teriflunomide) [108, 109].
Different classes of drugs are associated with different levels of risk. Compared to non-MS individuals of the same age and sex, MS patients who received GA and IFN-β1 via DMTs had a 50% higher risk of all serious infections (defined as an infection requiring hospitalization). the anti-CD20 antibody rituximab In comparison to GA and IFN-β1 dramatically increased the rate of overall severe infection in MS patients [110]. However, some believed that DMTs might protect against COVID-19 by reducing the cytokine-storm-like reactions [110, 111]. Additionally, several DMTs (such as GA, fumaric acid, and fingolimod) are linked to an upsurge in the expression of circulating natural killer cells, which could lead to a more effective defense against COVID-19 [112].
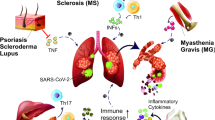
It is impossible to draw definite conclusions regarding the effect of medication and the severity of the infection in these patients. Figure 1 depicts the possible neurological damage as a result of COVID-19 infection (Fig. 1).
SARS-COV 2 potential for neurological disease exacerbation. Two potential neural pathways for SARS-COV 2 entry into the brain are by infection of the olfactory bulb or interactions with the eyes and oral mucosa. SARS-CoV-2 may transmit infection along blood–brain barrier (BBB) endothelial cells, blood-cerebrospinal fluid barrier epithelial cells in the choroid plexus, or it may enter the body through the CNS by making use of inflammatory cells as a "Trojan horse" [113]. Expression of IL-6, TNF-α, and other proinflammatory cytokines in a cytokine storm increased due to Viral presence, also glial inflammatory response may lead to damaged oligodendrocytes and BBB disruption, providing a second way for CNS invasion and lymphocyte infiltration [114]
COIVD-19 outcomes in children with autoimmune neurological diseases
Patients with autoimmune neurological illnesses diagnosed with COVID-19 are currently the subject of extensive data gathering. However, these samples have not yet reached statistical significance [115]. Previously, viral infections were linked to several autoimmune neurological conditions, most notably MS [116]. Relapses and/or worsening neurological symptoms are common effects of infections, which also significantly increase morbidity and contribute to the exacerbation of the disease [117, 118]. Upper respiratory viral infections, including infections with coronaviruses, have been shown to raise the possibility of relapse in people with MS [119, 120]. Coronaviruses account for 10 to 30 percent of these infections [119, 121].
Among the 404 patients with MS and SARS-CoV-2 infection enrolled in the prospective cohort study conducted by Garjani et al., 2021, 230 patients (57%) reported worsening symptoms of their MS. 207 patients reported a worsening of pre-existing symptoms, 82 reported the emergence of new MS symptoms, and 59 reported both [122]. To date, outcomes of COVID-19 in pediatrics with Autoimmune neurological diseases are largely unknown. Children frequently experience mild illness, because age is an established risk factor for serious illness [123].
In a small subgroup of patients with pediatric-onset MS who had been exposed to the SARS-CoV-2 virus or who had developed COVID-19, neither group required hospitalization nor respiratory support and reported no or mild symptoms. Additionally, they stated that comorbidities, neurological impairments, and DMTs had no influence on the outcomes of COVID-19 [115].
It has also been shown that COVID-19's extreme immunological activation and systemic stress may contribute to a higher frequency of relapses in people with MS [124, 125]. Also, The number of relapses in the pre-defined at-risk period was compared with the previous two years in a retrospective analysis of 41 patients with relapsing–remitting MS. The results revealed that COVID-19 may cause an exacerbation of MS [119].
Interestingly, children account for 30% of COVID-19-related ADEM cases reported so far. MIS-C, an entity associated with ADEM-like illnesses, is supposed to manifest 10 to 54 days after COVID-19 infection [93, 126, 127].
Henriques et al. reported that a 12-year-old girl with ADEM, severe cervical spinal cord myelopathy, and concurrent COVID-19 infection, finally evolved with partial clinical and neurological improvement and was subsequently discharged [128]. There were cases of younger children as well, for instance, a 17-month-old child who developed ADEM two weeks following COVID-19 pneumonitis and was finally able to fully recover after undergoing an intense immunomodulatory medication course [129]. The prognosis can vary. For instance, out of 38 pediatric patients with neuro-COVID-19, four passed away very quickly from fulminant systemic co-infections, and one child had a presumptive diagnosis of ADEM [126]. The definite underlying determinant of the prognosis is not yet discovered. However, investigations are ongoing. For example, children with monophasic or relapsing CNS demyelinating illnesses that include the optic, cerebral, and spinal structures and have overlapping clinical symptoms are reported to have myelin oligodendrocyte glycoprotein (MOG) antibody-associated disorders [130, 131]. In rare cases, COVID-19 infection might potentially cause an anti-MOG illness relapse [132].
Studies showed that the majority of COVID-19-positive neuromyelitis optica spectrum disorder (NMOSD) patients had moderate illness manifestations. However, compared to the general population, NMOSD patients had significantly greater probabilities of being admitted to the hospital and the critical care unit. Additionally, SARSCoV2 infection has increased the incidence of NMOSD relapses. Comorbidities such as Hypertension, obesity, diabetes, and dyslipidemias are the only predictor found to be associated with a worse COVID-19 outcome in patients with NMOSD (OR = 6.0, 95% CI: 1.79–19.98) [133]. However, some other reports show patients present similar incidence, risk factors, and outcomes for COVID-19 as the general population [134,135,136].
Treatment risks and advantages for patients with these disorders must be weighed against the degree to which disease-modifying medications restrict antiviral host immunity. As was previously noted, several immunotherapies are available for different neuroimmunological diseases [116].
Although there are controversial data, it can be concluded that higher disability status pre-existing comorbidities, patients with progressive forms, and a longer disease duration increased the risk of a more severe COVID-19 course of disease [122, 136, 137].
Other autoimmune disorders
COVID-19 in children with inflammatory bowel diseases (IBD)
Among individuals with inflammatory bowel diseases (IBD), COVID-19 susceptibility and disease progression are yet unknown, and there is a scarcity of epidemiological data on the subject [138]. We should anticipate a higher chance of SARS-CoV-2 infection and/or a worsening prognosis in patients with IBD. These patients have attenuated immune systems and an increased risk of infection due to the immunosuppressive medications they take [139]. Also, excessive cytokine production raises the ACE2 [140]. Thus, the gut mucosa of these patients exhibits an increased expression of ACE2 [141] and a subsequent rise in serum ACE2 levels (as well as Ang1–7 and the ACE2: ACE ratio) [142]. In the blood, this might serve as a protective factor by serving as the virus receptor’s rival which lowers the viral load that would otherwise infect the host. As both mucosal and serum ACE2 expression seems to be elevated in IBD and it is an important question as to how exactly ACE2 affects COVID-19 unfavorable consequences in IBD patients given its crucial function in enabling the virus' entrance into host cells [141,142,143]. In addition, given that immunosuppressive medication is frequently used by IBD patients, and subsequent immune system dysregulation, distinct COVID-19 forms in these individuals compared to the general population could be anticipated [144].
Few papers that have been published so far do not indicate an elevated incidence of COVID-19 in people with pediatric inflammatory bowel illness [139]. According to the Surveillance Epidemiology of Coronavirus under Research Exclusion (SECURE)-IBD registry, there have been 1760 instances of COVID-19 in IBD patients (85 of them are registered in Italy), of which 497 required hospitalization (28%) and 63 passed away (4%) [145]. According to a systematic study of IBD patients with COVID-19, 28 (11.4%) out of 246 patients required ICU care, 26 (3.7%) out of 697 patients required mechanical breathing, and 29 (3.8%) out of 796 patients died because of COVID-19 [146]. A SECURE-IBD registry preliminary report on the initial 525 patients from 33 countries reported that advanced age, the presence of at least two comorbidities, the administration of systemic steroids or sulfasalazine/5-aminosalicylate as risk factors of a worse course of the SARS-Cov-2 virus infection [147]. According to another study by Carparelli et al., out of 600 IBD adult patients analyzed, including pediatric patients, COVID-19 was diagnosed in 25 patients, none of whom were under the age of 18 years. This suggests that the incidence rate of COVID-19 is lower in children. Symptoms were frequently missing or barely noticeable. There was no recorded death [148].
A greater prevalence of hospitalization was associated with the use of corticosteroids (29 vs. 8%) and sulfasalazine/mesalazine medication (57% of inpatients vs. 21% of outpatients). In addition, exactly like in adults, the use of TNF antagonists alone was associated with a lower chance of hospitalization in pediatric patients (7% of hospitalized patients vs. 51% of non-hospitalized). [149]. Severe COVID-19 may be associated with clinically active IBD, especially in younger people. Controlling IBD disease is essential to prevent harmful COVID-19 effects, particularly through medication compliance and methods to lower the chance of COVID-19 infection in individuals with active IBD (i.e. isolation, vaccination) [150]. Moderate/severe disease activity was linked to an increased risk of hospitalization in a study of 209 pediatric IBD patients from the SECURE-IBD registry. However, the sample size was too limited to allow for the adjustment of confounding factors, and the outcomes like ICU admission and death were too few to examine. Additionally, it has been noted that children receiving biologics and/or other immune-suppressive treatments had a low risk of developing severe COVID-19 [149].
MIS-C, also called pediatric multi-system inflammatory syndrome temporally related to SARS CoV-2 (PMIS or PIMS-TS), is a potentially serious illness in children that appears to be a delayed, post-infectious complication of COVID-19 infection. The most common symptoms in children with PIMS-TS/MIS-C are gastrointestinal ones. Hence it could be difficult to distinguish this condition from an inflammatory bowel disease (IBD) flare-up. Data have demonstrated that in patients with underlying IBD, the primary cause of treatment and diagnostic difficulties in PIMS-TS/MIS-C is the similarity of clinical presentations. Children who have recovered from PIMS-TS/MIS-C require long-term follow-up to determine their risk of developing autoimmune diseases [151]. Other studies showed that despite continued immunomodulatory therapy, children with IBD and symptomatic or asymptomatic SARS-COV-2 infection could establish a protective humoral response against SARS-CoV-2 that is comparable to their peers without the disease [152, 153]. IBD treatment should not be stopped during the pandemic, according to current recommendations, as the risk of exacerbations outweighs the risk of any COVID-19 consequences [154, 155]. This is particularly for children, as in the first wave of the COVID-19 pandemic, illness flare-ups occurred in 21–23% of pediatric patients who had discontinued or temporarily paused their biological treatment [156].
Although there is a growing interest in the connection between COVID-19 and IBD, there are still some problems that need to be addressed in further in-depth research with larger patient cohorts, particularly in pediatric populations. The risk of infection or the severity of the disease does not seem to be higher for IBD patients. However, further research is needed to attain more certainty because the findings of the published studies so far are in disagreement.
COVID-19 in children with celiac disease
Given the strong expression of ACE2 and TMPRSS2 in the intestinal enterocytes, an increasing body of evidence supports the intestinal tropism of SARS-CoV-2. In fact, COVID-19 stimulates the production of a "cytokine storm" in the intestinal mucosa, which results in epithelial destruction and enhanced barrier permeability, allowing gliadin to pass through the intestinal lamina [157]. Impairment of the intestinal barrier causes the translocation of microbial elements, including microbial-associated molecular patterns (MAMPs), which in turn trigger an inflammatory immune response by TLR-expressing cells of the mesentery fat (primarily macrophages and adipocytes) and can thus enter the bloodstream [158]. These results support the idea that intestinal cells may contribute to an increase in SARS-CoV-2 viremia [157].
The etiology of many autoimmune illnesses has been linked to increased intestinal permeability because this particular permeability results in irregularities in the movement of components that might elicit certain autoimmune reactions [159]. Gliadin causes an autoimmune systemic illness called CD in people who are genetically prone to it (HLA DQ2 and DQ8) [160], which is associated with a higher risk of viral infections [161]. One of the key roles in the pathogenesis of CD is the disruption of the intestinal barrier [160]. CD Children and adults have both been described as being infected during the COVID-19 pandemic [162, 163]. There have been new proposed CD diagnostic methods [164], worse clinical status [165], and a decreased diagnostic rate described in CD patients during the COVID-19 pandemic [166].
CD patients, particularly untreated individuals, may be more susceptible to infections as well as viral diseases. It has been postulated that increased expression of CD4, CD25, and FOXP3 as anti-inflammatory markers in CD patients might be of benefit to reducing the severity of COVID-19 disease. Nevertheless, because of their elevated expression of IL-6, untreated CD patients may be more susceptible to severe COVID-19 if they contract the SARS-CoV-2 virus [167]. Future research involving CD patients infected with COVID-19 will need to demonstrate that increased expression of anti-inflammatory markers in these patients can reduce the severity of COVID-19. It is shown that CD patients with COVID-19 do not have a higher risk of death or hospitalization [168]. In Sweden, a recent population-based study of CD patients revealed no elevated risk of COVID-19-related outcomes [169]. None of the 387 pediatric CD patients in an Italian study experienced respiratory failure, developed pneumonia, needed oxygen therapy, or needed to be hospitalized. Additionally, compared to the general population, a significant rise in COVID-19 incidence was not indicated [163]. Hospitalization and mortality rates for CD patients who also have COVID-19 were 12% and 2.5%, respectively, according to a global registry of health professionals [168]. Moreover, increased age and new gastrointestinal symptoms in CD patients may raise their risk of adverse COVID-19 outcomes, similarly the patients without CD [170, 171].
COVID-19 in children with psoriasis
Psoriasis is a papulosquamous, immune-mediated, chronic skin condition that affects 125 million individuals worldwide [172]. A well-known cause of psoriasis, particularly guttate psoriasis in children, is viral and bacterial infections which can either cause psoriasis de novo or ignite an aggravation of psoriasis [173,174,175]. Similarly, SARSCoV-2 may exacerbate psoriasis [176,177,178]. The skew towards an excessive inflammatory cytokine milieu in psoriasis, notably tumor necrosis factor- and IL-17, may be responsible for the severe inflammation and tissue destruction seen in bacterial and viral infections [179, 180]. It was found that pro-inflammatory cytokines produced in excess cause immune response dysregulation and pathological inflammatory alterations connected to septic shock [181].
Following 69,315 patients with psoriasis, Yiu et al. [182] found a 36% increased risk of being hospitalized and a 33% increased risk of death because of serious infections among patients with psoriasis relative to matched controls. A controlled retrospective cohort study followed 25,742 psoriasis patients and revealed a higher chance of acquiring severe infections (adjusted hazard ratio 2.08; 95% CI 1.96–2.22) [183]. Similarly, a cohort of 199,700 psoriasis patients was found to have an increased risk of serious infections (adjusted hazard ratio 1.21; 95% CI 1.18–2.23) [184].
Apremilast is an oral phosphodiesterase-4 inhibitor approved for the treatment of chronic plaque psoriasis, psoriatic arthritis and Behcet’s disease [185]. Given that apremilast inhibits the expression of pro-inflammatory cytokines known to be released with SARS-CoV-2, such as tumor necrosis factor-alpha (TNF a), interleukin (IL)-17, and IL-23 [186], it is possible that using this oral small molecule may reduce the risk of a cytokine storm associated with SARS-CoV-2 infections. Patients using apremilast may therefore have a lower risk of experiencing serious complications with COVID-19 [187, 188]. Apremilast exhibited the lowest SARS-CoV-2 infection rate when compared to biologic treatments in a Spanish cohort of psoriasis patients [189]. Adults are the focus of the majority of published data on the usage of systemic psoriasis therapies and COVID-19 outcomes. Old age, male sex, non-white ethnicity, and comorbidities (mostly chronic lung diseases) are the same risk factors for worse COVID-19 outcomes in adult psoriasis patients as they are in the general population, according to Mahil et al. (PsoProtect registry) [190].
Biologic treatments, cyclosporine, and methotrexate, among other systemic psoriasis medications, are known to make both adults and children more susceptible to infections [191]. It has been revealed that compared to children receiving biological medications, children receiving non-biologic systemic therapy (P = 0.02) and those not receiving systemic treatment (P = 0.006) had significantly longer COVID-19 symptom durations (6.5 days on average). Additionally, it is noted that the six hospitalized children were treated with non-biologic systemic medications, methotrexate in particular (P = 0.03), more frequently than the others (P = 0.01). In 17 patients (15.2%) after COVID-19, psoriasis got worse. In the month after COVID-19, nine children (8%) developed psoriasis (P = 0.01) [192]. The COVID-19 treatment course in psoriasis patients of all ages has been evaluated by the PsoProtect registry. It demonstrated that utilizing biologics did not make psoriasis patients more likely to be hospitalized. Few pediatric patients were included in PsoProtect and other investigations, and no pediatric-specific subgroup analysis has yet been carried out [39, 178, 181, 187,188,189].
In a French study, the first and second waves of the pandemic were separately analyzed as they examined the COVID-19 outcome in individuals with psoriasis from the national health insurance database. For both waves, no difference in mortality between patients receiving biologic vs. non-biologic systemic medications was reported. During the initial wave, they observed an elevated risk of hospitalization for individuals using non-biologic systemic medications [193].
Studies observed no evidence of an elevated risk of severe COVID-19 in patients receiving biologic therapy in this multinational registry of psoriatic infants, children, and adolescents from 14 countries across three continents who developed COVID-19. Biologic treatment for psoriasis in children does not seem to increase their chance of getting COVID-19 severe. For some, COVID-19 caused psoriasis to develop or worsen in people who already had it [192].
COVID-19 treatment in children with autoimmune disorders
Due to the increased risk of COVID-19 infection, the likelihood of serious COVID-19 consequences, and the potential for pre-existing illness flares, patients with autoimmune diseases and their caregivers are concerned about how well these patients are being treated. The underlying autoimmune diseases’ pathophysiology and severity, as well as the received medications, can impact the Covid-19 outcomes in patients with autoimmune diseases [194].
Children with autoimmune disorders may present different symptoms and require specific prevention plans or therapies, and medical professionals must remain informed about these treatments to provide the best care to their patients. For instance, while hydroxychloroquine has been widely administered for COVID-19 treatment, the management of SARS-CoV-2-infected T1DM patients has demonstrated that it can lead to a reduction in insulin breakdown and, subsequently, hypoglycemia [195]. Nevertheless, antiviral medications like ritonavir and lopinavir have been shown to decrease glycemic control and result in hyperglycemia [196]. Significant hyperglycemia can also result from the use of glucocorticoids, which are widely used as a part of the COVID-19 treatment regimen for hospitalized patients [195].
Outpatient SARS-CoV-2 treatment includes Monoclonal antibodies, remdesivir, and oral medicines like nirmatrelvir/ritonavir and molnupiravir [197,198,199,200]. Studies have shown that patients with systemic autoimmune rheumatic diseases (SARD) can benefit from receiving monoclonal antibodies and also from oral antiviral medications. However, there are some concerns raised about a higher risk of COVID-19 rebound following the administration of the latter [201].
Despite these concerns, a cohort study of SARD patients with COVID-19 found that a rebound has occurred in only 8% of SARD patients who received oral outpatient therapy. Furthermore, none of these patients ended up demonstrating severe symptoms or were hospitalized as a result of the COVID-19 rebound. Compared to no outpatient treatment, antiviral or monoclonal antibody outpatient treatment was linked with 88% lower odds of severe COVID-19 infection [201]. Another investigation revealed that both oral antiviral therapies (molnupiravir and nirmatrelvir/ritonavir) had positive outcomes and adequate safety profiles among a high-risk SARD population [202].
On the other hand, Many immunomodulatory therapies that are routinely used for autoimmune diseases, including tocilizumab, which is often used to treat autoimmune diseases, may help control the immune response against SARS-CoV-2 [203]. Antimalarial medications chloroquine and hydroxychloroquine, have immunomodulatory properties and are also used to treat autoimmune conditions such as SLE and RA. Both medications are reported to prevent the fusion of the SARS-CoV-2 coronavirus with host cell membranes. Moreover, chloroquine prevents the cellular ACE2 receptor from being glycosylated, which might prevent SARS-CoV-2 from attaching to its cellular receptor. Both chloroquine and hydroxychloroquine can also inhibit the movement of SARS-CoV-2 from early endosomes to endolysosomes in vitro, which is necessary for the release of the viral genome [204, 205]. One study on immunosuppressive medication in psoriasis patients with COVID-19 found that those taking apremilast have a reduced incidence of infection. It is important to investigate the bidirectional impact of COVID-19, as an immunomodulatory acute condition, and immunomodulatory drugs. Studying these can help to investigate if continuing these drugs for autoimmune patients, infected or at risk of COVID-19, would be safe. These investigations, for instance in the case of chloroquine and hydroxychloroquine, might even lead to the usage of certain immunomodulatory drugs for COVID-19 treatment in the normal population [189].
In conclusion, although it is well known that those with pre-existing autoimmune disorders are at higher risk for severe complications from COVID-19, it is important to conduct further studies to understand the potential differences of common anti-COVID-19 therapies in the treatment of the virus in this underrepresented pediatric population.
Future perspective of pediatrics autoimmune diseases post-COVID-19 pandemic
COVID-19 and comorbidities created a vicious cycle that dramatically increases mortality and morbidity in affected patients. The pathogenesis of both COVID-19 and autoimmune diseases entails an active immune system response. In addition to lymphopenia, COVID-19 can affect T-cell function, upregulate inhibitory immune checkpoint expression, and exacerbate inflammatory cytokines [190]. Patients with COVID-19 may also exhibit autoantibodies, which are common in autoimmune diseases. Furthermore, following the COVID-19 infection, some patients have developed autoimmune illnesses such as Guillain–Barre syndrome or SLE [127, 206]. Actually, severe COVID-19 has been documented to result in cytokine storms due to the overproduction of pro-inflammatory cytokines such as IL-6.
The best way to treat individuals with immune-mediated disorders that require systemic medications during the COVID-19 pandemic is a major source of concern for the medical community, for instance, regarding the fact that immunosuppressive therapy can compromise antiviral immunity (Fig. 2). In some studies, the risk of poor COVID-19 outcomes related to the use of biological medications in inflammatory illnesses such as psoriasis, rheumatologic, and intestinal diseases in people has been evaluated. Patients with severe asthma who were receiving biologic therapy had a more severe COVID-19 course than people in the general population [207]. The analysis also showed that Immune-mediated inflammatory disorders were associated with a greater rate of COVID-19 mortality and hospital admissions [208]. However, data specifically about autoimmune disorders are still needed.
Adverse effects of immunosuppression that may influence the course of COVID-19. Reducing the immune systems' capacity to fight infections is one of the most important side effects to be concerned about in immunocompromised patients. Immunosuppressive medications have anti-inflammatory and immunomodulatory effects by preventing the synthesis of pro-inflammatory cytokines, lowering leucocyte trafficking, and causing T-lymphocyte apoptosis. These drugs stop the immune system from battling the pathogen. Hence, in the early phases of COVID-19, they could be hazardous. There is currently disagreement over the overall impact of immunosuppressive medications in patients with COVID-19
Due to uncertainties in epidemiologic data on children, there is still much to learn about COVID-19 manifestations in children with autoimmune diseases. The patient might ultimately experience severe outcomes as a result of the consequences, including multiple organ failure, shock, acute respiratory distress syndrome, heart failure, arrhythmias, renal failure, and, in the worst cases, death. Consequently, better management with special consideration must be given to these patients. There is still a need for more research in order to identify the specific COVID-19 characteristics that differ between children and adults.
The preliminary results from the ongoing trials for the COVID-19 vaccination in children have demonstrated reassuring efficacy and tolerance. The balance of risk and benefit of immunization in this age group is controversial, mostly due to the relatively low risk of COVID-19 infection in children and the lack of confidence regarding the relative effects of vaccination and disease [209, 210]. The duration of the immune responses and protection provided by vaccine regimens in pediatric patients with underlying disorders call for further research, as well.
Conclusion
The COVID-19 global pandemic posed severe health risks, particularly for people with pre-existing medical disorders. Initial concerns that patients with neuro-immune diseases were at a higher risk of developing a severe COVID-19 infection stemmed from differences in immune function caused by the conditions or treatments. Numerous studies on adults and young adolescents revealed that these groups of patients had worse COVID-19 outcomes. A relatively small number of studies reported such an observation in the pediatric age group. Given the rarity of pediatric cases, it is understandable that only case reports and studies with small sample sizes have been published.
It would be interesting to see if the presence of pre-existing immune-mediated diseases or prior use of immunomodulatory drugs influences the phenotype of COVID-19. While some studies found that patients with certain immune-mediated and autoimmune diseases had a higher risk of infection and a more aggressive course of SARS-CoV-2 infection, other studies disagreed. COVID-19 outcomes may be worse for patients receiving immunomodulatory therapies, particularly those with severe comorbidities. On the other hand, it has been proposed that the lung damage caused by SARS-CoV-2 is the result of an overactive immune system and that immunosuppressive medication may benefit some patients.
Finally, it is unclear how immune-related illnesses will affect the progression of COVID-19. The infection risk and prognosis of COVID-19 in patients with autoimmune diseases are still being debated, but patient adherence to medication regimens to prevent autoimmune disease flare-ups is strongly advised.
Availability of data and materials
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Abbreviations
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- RMD:
-
Rheumatic and musculoskeletal diseases
- OL:
-
Oligodendrocyte
- MIS-C:
-
Multisystem inflammatory syndrome in children
- PIMS-TS:
-
Pediatrics multisystem inflammatory syndrome temporally associated with COVID-19
- HRSV:
-
Human respiratory syncytial virus
- bDMARD:
-
Biological Disease-modifying antirheumatic drugs
- MS:
-
Multiple sclerosis
- ADEM:
-
Acute disseminated encephalomyelitis
- ARP:
-
Pre-defined at-risk period
- SLE:
-
Systemic lupus erythematosus
- RA:
-
Rheumatoid arthritis
- SARD:
-
Systemic autoimmune rheumatic diseases
- TIDM:
-
Type-1 Diabetes Mellitus
- T2DM:
-
Type-2 Diabetes Mellitus
- JIA:
-
Juvenile idiopathic arthritis
- FMF:
-
Familial Mediterranean fever
- jSLE:
-
Juvenile systemic lupus erythematosus
- CTD:
-
Connective tissue disease
- AID:
-
Autoinflammatory disease
- ROCM:
-
Rhino-orbito-cerebral mucormycosis
References
Mercer TR, Salit M (2021) Testing at scale during the COVID-19 pandemic. Nat Rev Genet 22(7):415–426. https://doi.org/10.1038/s41576-021-00360-w
Nalbandian A et al (2021) Post-acute COVID-19 syndrome. Nat Med 27(4):601–615. https://doi.org/10.1038/s41591-021-01283-z
Kumar S, Tripathi T (2020) One year update on the COVID-19 pandemic: Where are we now? Acta Trop. 2021;214:105778. https://doi.org/10.1016/j.actatropica.2020.105778
Howard-Jones AR et al (2022) COVID-19 in children. II: Pathogenesis, disease spectrum and management. J Paediatr Child Health 58(1):46–53. https://doi.org/10.1111/jpc.15811
Brito-Zerón P, Sisó-Almirall A, Flores-Chavez A, Retamozo S, Ramos-Casals M (2021) SARS-CoV-2 infection in patients with systemic autoimmune diseases. Clin Exp Rheumatol 39(3):676–687
Nilea SH, Nilea A, Qiua J, Lib L, Jiac X, Kaia G (2020) COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev 54(January):66–70
WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. https://covid19.who.int/. Accessed 14 Mar 2023
Siebach MK, Piedimonte G, Ley SH (2021) COVID-19 in childhood: Transmission, clinical presentation, complications and risk factors. Pediatr Pulmonol 56(6):1342–1356. https://doi.org/10.1002/ppul.25344
Mehraeen E et al (2021) COVID-19 in pediatrics: The current knowledge and practice. Infect Disord Drug Targets. https://doi.org/10.2174/1871526521666210929121705
Child mortality and COVID-19 - UNICEF DATA. https://data.unicef.org/topic/child-survival/covid-19/. Accessed 10 Jun 2022
de Souza TH, Nadal JA, Nogueira RJN, Pereira RM, Brandão MB (2020, Aug) Clinical manifestations of children with COVID-19: a systematic review. Pediatr Pulmonol 55(8):1892–1899. https://doi.org/10.1002/ppul.24885. Epub 2020 Jun 15. PMID: 32492251; PMCID: PMC7300659
Choi JH, Choi SH, Yun KW (2022) Risk Factors for Severe COVID-19 in Children: A Systematic Review and Meta-Analysis. J Korean Med Sci 37(5):1–14. https://doi.org/10.3346/JKMS.2022.37.E35
Tsankov BK et al (2021) Severe COVID-19 Infection and Pediatric Comorbidities: A Systematic Review and Meta-Analysis. Int J Infect Dis 103:246–256. https://doi.org/10.1016/j.ijid.2020.11.163
Williamson EJ et al (2020) OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature 584(7821):430–436. https://doi.org/10.1038/s41586-020-2521-4
Akiyama S, Hamdeh S, Micic D, Sakuraba A (2021) Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: A systematic review and meta-analysis. Ann Rheum Dis 80(3):384–391. https://doi.org/10.1136/annrheumdis-2020-218946
Deb P, Zannat KE, Talukder S, Bhuiyan AH, Jilani MSA, Saif-Ur-Rahman KM (2022) Association of HLA gene polymorphism with susceptibility, severity, and mortality of COVID-19: A systematic review. Hla 99(4):281–312. https://doi.org/10.1111/tan.14560
Sawalha AH, Zhao M, Coit P, Lu Q (2020) Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clin Immunol 215(April):108410. https://doi.org/10.1016/j.clim.2020.108410
Gough SCL, Simmonds MK (2007) The HLA Region and Autoimmune Disease: Associations and Mechanisms of Action. Curr Genomics pp 453–465
Freites Nuñez DD et al (2020) Risk factors for hospital admissions related to COVID-19 in patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 79(11):1393–1399. https://doi.org/10.1136/annrheumdis-2020-217984
Tan EH et al (2021) COVID-19 in patients with autoimmune diseases: Characteristics and outcomes in a multinational network of cohorts across three countries. Rheumatol 60(SI):SI37–SI50. https://doi.org/10.1093/rheumatology/keab250
Brandel J-P et al (2020) Covid-19 in Immune-Mediated Inflammatory Diseases — Case Series from New York To. N Engl J Med 383(1):83–85. https://doi.org/10.1056/nejmc2000687
Craig ME, Hattersley A, Donaghue KC (2009) Definition, epidemiology and classification of diabetes in children and adolescents. Pediatr Diabetes 10(SUPPL. 12):3–12. https://doi.org/10.1111/j.1399-5448.2009.00568.x
Los E, Wilt AS (2023, Feb 5) Diabetes mellitus type 1 in children. In: StatPearls [Internet]. Treasure Island (FL), StatPearls Publishing; 2023 Jan–. PMID: 28722947
Codo AC et al (2020) Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab 32(3):437-446.e5. https://doi.org/10.1016/j.cmet.2020.07.007
Long H et al (2022) Plasma glucose levels and diabetes are independent predictors for mortality in patients with COVID-19. Epidemiol Infect 150(T Cd). https://doi.org/10.1017/S095026882200022X
Critchley JA, Carey IM, Harris T, DeWilde S, Hosking FJ, Cook DG (2018) Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care 41(10):2127–2135. https://doi.org/10.2337/dc18-0287
Elbarbary NS, dos Santos TJ, de Beaufort C, Agwu JC, Calliari LE, Scaramuzza AE (2020) COVID-19 outbreak and pediatric diabetes: Perceptions of health care professionals worldwide. Pediatr Diabetes 21(7):1083–1092. https://doi.org/10.1111/pedi.13084
Wu L, Girgis CM, Cheung NW (2020) COVID-19 and diabetes: Insulin requirements parallel illness severity in critically unwell patients. Clin Endocrinol (Oxf) 93(4):390–393. https://doi.org/10.1111/cen.14288
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395(10229):1033–1034. https://doi.org/10.1016/S0140-6736(20)30628-0
Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H (2020) Correspondence Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. Nejm38(1):1–3
Sestan M et al (2018) Virus-induced interferon-γ causes insulin resistance in skeletal muscle and derails glycemic control in obesity. Immunity 49:164–177.e6
Weyer C, Bogardus C, Mott DM, Pratley RE (1999) The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 104(6):787–794. https://doi.org/10.1172/JCI7231
Lim S, Bae JH, Kwon HS, Nauck MA (2021) COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol 17(1):11–30. https://doi.org/10.1038/s41574-020-00435-4
Kompaniyets L et al (2021) Underlying Medical Conditions Associated with Severe COVID-19 Illness among Children. JAMA Netw Open 4(6):1–14. https://doi.org/10.1001/jamanetworkopen.2021.11182
Arachchillage DRJ, Laffan M (2020) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18(5):1233–1234. https://doi.org/10.1111/jth.14820
Cadegiani F, Luiz P, Da Silva H (2020) Risk of Complications in Children With Type 1 Diabetes and Covid-19. 4:1210. https://doi.org/10.1210/jendso/bvab048
Nimri R et al (2022) Symptoms and Glycemic Control in Young People With Type 1 Diabetes Following SARS-CoV-2 Infection: An Observational Study. J Clin Endocrinol Metab May:1–9. https://doi.org/10.1210/clinem/dgac288
Coronavirus infection (COVID-19) and children with diabetes - International Society for Pediatric and Adolescent Diabetes. https://www.ispad.org/page/CoronavirusinfectionCOVID-19. Accessed 14 Aug 2022
Bornstein SR et al (2021) Practical recommendations of the German Diabetes Society for the management of diabetes in patients with COVID-19. Diabetologe 17(1):36–41. https://doi.org/10.1007/s11428-020-00715-7
Zhu L et al (2020) Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab 31(6):1068-1077.e3. https://doi.org/10.1016/j.cmet.2020.04.021
Diwakar J et al (2021) First report of COVID-19-associated rhino-orbito-cerebral mucormycosis in pediatric patients with type 1 diabetes mellitus. J Med Mycol 31(4):101203. https://doi.org/10.1016/j.mycmed.2021.101203
Scaramuzza AE, Rabbone I, Maffeis C, Schiaffini R (2021) Seasonal flu and COVID-19 recommendations for children, adolescents and young adults with diabetes. Diabet Med 38(1):19–20. https://doi.org/10.1111/dme.14427
Alonso GT et al (2021) Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes 13(8):681–687. https://doi.org/10.1111/1753-0407.13184
Vasconez WA, Bustamante Escobar CL, Agarwal N, Solano JP, Sanchez JE (2021) Severe Diabetic Ketoacidosis in a Child with Type-1 Diabetes, Asthma, and COVID-19. J Pediatr Intensive Care 10(3):232–234. https://doi.org/10.1055/s-0040-1713164
Naguib MN, Raymond JK, Vidmar AP (2021) New onset diabetes with diabetic ketoacidosis in a child with multisystem inflammatory syndrome due to COVID-19. J Pediatr Endocrinol Metab 34(1):147–150. https://doi.org/10.1515/jpem-2020-0426
Alkadhem S et al (2020) Multisystem inflammatory syndrome in children with DM type I: a case report from Saudi Arabia. Int J Med Dev Ctries 4(September 2020):2023–2026. https://doi.org/10.24911/ijmdc.51-1599813373
Feldstein LR et al (2020) Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med 383(4):334–346. https://doi.org/10.1056/nejmoa2021680
Dufort EM et al (2020) Multisystem Inflammatory Syndrome in Children in New York State. N Engl J Med 383(4):347–358. https://doi.org/10.1056/nejmoa2021756
States U et al (2020) COVID-19 – Associated Multisystem Inflammatory Syndrome in Children (vol. 69, no. 32, pp 1074–1080)
Gregory JM, Moore DJ (2022) Age and Hospitalization Risk in People With Type 1 Diabetes and COVID-19: Data From the T1D Exchange Surveillance Study. J Clin Endocrinol Metab 107(4):E1763–E1764. https://doi.org/10.1210/clinem/dgab871
Sherif EM, Elhenawy YI, Matter RM, Aly HH, Thabet RA, Fereig YA (2021) Clinical characteristics and outcome of hospitalized children and adolescent patients with type 1 diabetes during the COVID-19 pandemic: Data from a single center surveillance study in Egypt. J Pediatr Endocrinol Metab 34(7):925–936. https://doi.org/10.1515/jpem-2021-0099
Verma A, Verma S, Dochania K, Vaswani ND (2021, Jul–Aug) Effect of COVID 19 second wave on children with type 1 diabetes mellitus in India. Diabetes Metab Syndr 15(4):102171. https://doi.org/10.1016/j.dsx.2021.06.008. Epub 2021 Jun 9. PMID: 34186360; PMCID: PMC8188778
Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM (2021) Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes 22(2):202–206. https://doi.org/10.1111/pedi.13158
Athreya BH (1996) Management of rheumatic diseases in children. Indian J Pediatr 63(3):305–321. https://doi.org/10.1007/BF02751523
Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE (2002) Frequency of infection in patients with rheumatoid arthritis compared with controls: A population-based study. Arthritis Rheum 46(9):2287–2293. https://doi.org/10.1002/ART.10524
Au K et al (2011) High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis 70(5):785–791. https://doi.org/10.1136/ARD.2010.128637
Danza A, Ruiz-Irastorza G (2013) Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. 22(12):1286–1294. https://doi.org/10.1177/0961203313493032
Sozeri B et al (2022) The clinical course of SARS-CoV-2 infection among children with rheumatic disease under biologic therapy: a retrospective and multicenter study. Rheumatol Int 42(3):469–475. https://doi.org/10.1007/s00296-021-05008-w
Filocamo G, Minoia F, Carbogno S, Costi S, Romano M, Cimaz R (2021) Absence of severe complications from SARS-CoV-2 infection in children with rheumatic diseases treated with biologic drugs. J Rheumatol 48(8):1343–1344. https://doi.org/10.3899/jrheum.200483
Haslak F et al (2022) Asymptomatic SARS-CoV-2 seropositivity: patients with childhood-onset rheumatic diseases versus healthy children. Clin Rheumatol 41(5):1523–1533. https://doi.org/10.1007/s10067-022-06067-5
Haslak F et al (2022) Comparisons of Clinical Features and Outcomes of COVID-19 between Patients with Pediatric Onset Inflammatory Rheumatic Diseases and Healthy Children. J Clin Med 11(8):1–13. https://doi.org/10.3390/jcm11082102
Listing J, Gerhold K, Zink A (2013) The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatol (United Kingdom) 52(1):53–61. https://doi.org/10.1093/rheumatology/kes305
Lakota K et al (2021) COVID-19 in Association With Development, Course, and Treatment of Systemic Autoimmune Rheumatic Diseases. Front Immunol 11(January):1–13. https://doi.org/10.3389/fimmu.2020.611318
Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D (2020) Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents 55(4):105932. https://doi.org/10.1016/J.IJANTIMICAG.2020.105932
Kilian A et al (2020) Acute respiratory viral adverse events during use of antirheumatic disease therapies: A scoping review. Semin Arthritis Rheum 50(5):1191–1201. https://doi.org/10.1016/J.SEMARTHRIT.2020.07.007
Vastert SJ et al (2010) Mutations in the perforin gene can be linked to macrophage activation syndrome in patients with systemic onset juvenile idiopathic arthritis. Rheumatology 49(3):441–449. https://doi.org/10.1093/RHEUMATOLOGY/KEP418
Canna SW et al (2014) An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet 46(10):1140–1146. https://doi.org/10.1038/ng.3089
Balsa A et al (2019) Prevalence of Comorbidities in Rheumatoid Arthritis and Evaluation of Their Monitoring in Clinical Practice: The Spanish Cohort of the COMORA Study. Reumatol Clínica (English Ed) 15(2):102–108. https://doi.org/10.1016/J.REUMAE.2017.06.003
Ranganath VK et al (2013) Comorbidities are associated with poorer outcomes in community patients with rheumatoid arthritis. Rheumatology 52(10):1809–1817. https://doi.org/10.1093/RHEUMATOLOGY/KET224
Haşlak F, Yıldız M, Adrovic A, Barut K, Kasapçopur Ö (2020) Childhood rheumatic diseases and COVID-19 pandemic: An intriguing linkage and a new Horizon. Balkan Med J 37(4):184–188. https://doi.org/10.4274/balkanmedj.galenos.2020.2020.4.43
Conway R et al (2022) SARS–CoV-2 Infection and COVID-19 Outcomes in Rheumatic Diseases: A Systematic Literature Review and Meta-Analysis. Arthritis Rheumatol 74(5):766–775. https://doi.org/10.1002/art.42030
Opoka-Winiarska V et al (2022) Programmed Cell Death Protein-1 Upregulation in Response to SARS-CoV-2 in Juvenile Idiopathic Arthritis: A Case-Control Study. J Clin Med 11(14):1–11. https://doi.org/10.3390/jcm11144060
Walters HM et al (2022) Eroprevalence and clinical outcomes of SARS-CoV-2 in paediatric patients with rheumatic disease. Rheumatology (Oxford) 61(SI2):SI112–SI119. https://doi.org/10.1093/rheumatology/keab730
Clemente D et al (2021) Clinical characteristics and COVID-19 outcomes in a regional cohort of pediatric patients with rheumatic diseases. Pediatr Rheumatol 19(1):1–8. https://doi.org/10.1186/s12969-021-00648-5
Udaondo C et al (2022) Clinical course and seroprevalence of COVID-19 in children with rheumatic diseases—cross-sectional study from a reference centre in Spain. Clin Rheumatol 41(6):1779–1784. https://doi.org/10.1007/s10067-022-06186-z
Boyarchuk O, Predyk L, Yuryk I (2021) COVID-19 in patients with juvenile idiopathic arthritis: Frequency and severity. Reumatologia 59(3):197–199. https://doi.org/10.5114/reum.2021.107590
Koker O et al (2020) Does immunosuppressive treatment entail an additional risk for children with rheumatic diseases? A survey-based study in the era of COVID-19. Rheumatol Int 40(10):1613–1623. https://doi.org/10.1007/s00296-020-04663-9
Villacis-Nunez DS, Rostad CA, Rouster-Stevens K, Khosroshahi A, Chandrakasan S, Prahalad S (2021) Outcomes of COVID-19 in a cohort of pediatric patients with rheumatic diseases. Pediatr Rheumatol 19(1):1–8. https://doi.org/10.1186/s12969-021-00568-4
Demir F, Ulu K, Çağlayan Ş, Coşkuner T, Sözeri B (2021, Jan–Feb) Clinical course of COVID-19 in children with rheumatic disease under biologic therapy. Clin Exp Rheumatol 39 Suppl 128(1):36–37. Epub 2021 Feb 19. PMID: 33634781
Sengler C et al (2021) Clinical manifestations and outcome of SARS-CoV-2 infections in children and adolescents with rheumatic musculoskeletal diseases: Data from the National Paediatric Rheumatology Database in Germany. RMD Open 7(2). https://doi.org/10.1136/rmdopen-2021-001687
Akgün Ö et al (2022) Humoral response and safety of BNT162b2 mRNA vaccine in children with rheumatic diseases. Rheumatology pp 1–9. https://doi.org/10.1093/rheumatology/keac140
Opoka-Winiarska V, Grywalska E, Korona-Glowniak I, Matuska K, Malm A, Roliński J (2021) Seroprevalence of antibodies against sars-cov-2 in children with juvenile idiopathic arthritis a case-control study. J Clin Med 10(8):1–10. https://doi.org/10.3390/jcm10081771
Sözeri B, Demir F, Kalın S, Akkuş CH, Salı E, Çakır D (2021) Sars-cov-2 infection in children with rheumatic disease: Experience of a tertiary referral center. Arch Rheumatol 36(3):381–388. https://doi.org/10.46497/ArchRheumatol.2021.8603
Mao L et al (2020) Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol 77(6):683–690. https://doi.org/10.1001/JAMANEUROL.2020.1127
Ellul MA et al (2020) Neurological associations of COVID-19. Lancet Neurol 19(9):767–783. https://doi.org/10.1016/S1474-4422(20)30221-0
DeBiasi RL et al (2020) Severe COVID-19 in Children and Young Adults in the Washington DC Metropolitan Region. Open Forum Infect Dis 7(Suppl 1):S338. https://doi.org/10.1093/OFID/OFAA439.738
Christy A (2020) COVID-19: A Review for the Pediatric Neurologist. 35(13):934–939. https://doi.org/10.1177/0883073820939387
Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P (2020) Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 395(10237):1607–1608. https://doi.org/10.1016/S0140-6736(20)31094-1
Dugue R et al (2020) Neurologic manifestations in an infant with COVID-19. Neurology 94(24):1100–1102. https://doi.org/10.1212/WNL.0000000000009653
McAbee GN, Brosgol Y, Pavlakis S, Agha R, Gaffoor M (2020) Encephalitis Associated with COVID-19 Infection in an 11-Year-Old Child. Pediatr Neurol 109:94. https://doi.org/10.1016/j.pediatrneurol.2020.04.013
Chacón-Aguilar R, Osorio-Cámara JM, Sanjurjo-Jimenez I, González-González C, López-Carnero J, Pérez-Moneo B (2020) COVID-19: Fever syndrome and neurological symptoms in a neonate. An Pediatr 92(6):373. https://doi.org/10.1016/J.ANPEDE.2020.04.001
Bhatta S, Sayed A, Ranabhat B, Bhatta RK, Acharya Y (2020) “New-Onset Seizure as the Only Presentation in a Child With COVID-19. Cureus 12(6). https://doi.org/10.7759/CUREUS.8820
Hacohen Y et al (2020) Neurologic and Radiographic Findings Associated With COVID-19 Infection in Children. JAMA Neurol 77(11):1440–1445. https://doi.org/10.1001/JAMANEUROL.2020.2687
Vivanti AJ et al (2020) Transplacental transmission of SARS-CoV-2 infection. Nat Commun 11(1):1–7. https://doi.org/10.1038/s41467-020-17436-6
Schupper AJ, Yaeger KA, Morgenstern PF (2020) Neurological manifestations of pediatric multi-system inflammatory syndrome potentially associated with COVID-19. Child’s Nerv Syst 36(8):1579–1580. https://doi.org/10.1007/S00381-020-04755-8/FIGURES/1
Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203(2):631–637. https://doi.org/10.1002/PATH.1570
MacDougall M, El-Hajj Sleiman J, Beauchemin P, Rangachari M (2022) SARS-CoV-2 and Multiple Sclerosis: Potential for Disease Exacerbation. Front Immunol 13(April):1–22. https://doi.org/10.3389/fimmu.2022.871276
Lima M et al (2020) Unraveling the Possible Routes of SARS-COV-2 Invasion into the Central Nervous System. Curr Treat Options Neurol 22(11). https://doi.org/10.1007/s11940-020-00647-z
Lin C, Arevalo YA, Nanavati HD, Lin DM (2020) Racial differences and an increased systemic inflammatory response are seen in patients with COVID-19 and ischemic stroke. Brain Behav Immun Heal 8:100137. https://doi.org/10.1016/J.BBIH.2020.100137
Sormani MP et al (2021) DMTs and Covid-19 severity in MS: a pooled analysis from Italy and France. Ann Clin Transl Neurol 8(8):1738–1744. https://doi.org/10.1002/ACN3.51408
Esposito S, Di Pietro GM, Madini B, Mastrolia MV, Rigante D (2015) A spectrum of inflammation and demyelination in acute disseminated encephalomyelitis (ADEM) of children. Autoimmun Rev 14(10):923. https://doi.org/10.1016/J.AUTREV.2015.06.002
Filippi M, Rocca MA (2020) Acute Disseminated Encephalomyelitis. White Matter Dis pp. 109–125. https://doi.org/10.1007/978-3-030-38621-4_5
Hussein O, Minagar A (2017) Acute Disseminated Encephalomyelitis: Clinical Features, Pathophysiology, and Clinical Management. Inflamm Disord Nerv Syst pp 161–173. https://doi.org/10.1007/978-3-319-51220-4_7
Ishizu T et al (2006) CSF cytokine and chemokine profiles in acute disseminated encephalomyelitis. J Neuroimmunol 175(1–2):52–58. https://doi.org/10.1016/J.JNEUROIM.2006.03.020
Mangalmurti N, Hunter CA (2020) Cytokine Storms: Understanding COVID-19. Immunity 53(1):19–25. https://doi.org/10.1016/J.IMMUNI.2020.06.017
Hussein O, Abd Elazim A, Torbey MT (2020, Dec 15) Covid-19 systemic infection exacerbates pre-existing acute disseminated encephalomyelitis (ADEM). J Neuroimmunol 349:577405. https://doi.org/10.1016/j.jneuroim.2020.577405. Epub 2020 Sep 25. PMID: 33002725; PMCID: PMC7518115
Berger JR, Brandstadter R, Bar-Or A (2020) COVID-19 and MS disease-modifying therapies. Neurol - Neuroimmunol Neuroinflammation 7(4):761. https://doi.org/10.1212/NXI.0000000000000761
Winkelmann A, Loebermann M, Reisinger EC, Hartung HP, Zettl UK (2016) Disease-modifying therapies and infectious risks in multiple sclerosis. Nat Rev Neurol 12(4):217–233. https://doi.org/10.1038/nrneurol.2016.21
Rae-Grant A et al (2018) Comprehensive systematic review summary: Disease-modifying therapies for adults with multiple sclerosis. Neurology 90(17):789–800. https://doi.org/10.1212/WNL.0000000000005345
Luna G et al (2020) Infection Risks Among Patients With Multiple Sclerosis Treated With Fingolimod, Natalizumab, Rituximab, and Injectable Therapies. JAMA Neurol 77(2):184–191. https://doi.org/10.1001/JAMANEUROL.2019.3365
Novi G et al (2020) COVID-19 in a MS patient treated with ocrelizumab: does immunosuppression have a protective role? Mult Scler Relat Disord 42:102120. https://doi.org/10.1016/J.MSARD.2020.102120
Al-Ani M, Elemam NM, Hundt JE, Maghazachi AA (2020) Drugs for Multiple Sclerosis Activate Natural Killer Cells: Do They Protect Against COVID-19 Infection? Infect Drug Resist 13:3243. https://doi.org/10.2147/IDR.S269797
Keyhanian K, Umeton RP, Mohit B, Davoudi V, Hajighasemi F, Ghasemi M (2021) SARS-CoV-2 and nervous system: From pathogenesis to clinical manifestation. J Neuroimmunol 350:577436. https://doi.org/10.1016/j.jneuroim.2020.577436
Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L (2020) SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev 54:62. https://doi.org/10.1016/J.CYTOGFR.2020.06.001
Oncel I, Alici N, Solmaz I, Oge DD (2020) The Outcome of COVID-19 in Pediatric-Onset Multiple Sclerosis Patients
Hartung HP, Aktas O (2020) COVID-19 and management of neuroimmunological disorders. Nat Rev Neurol 16(7):347–348. https://doi.org/10.1038/s41582-020-0368-9
Willis MD, Robertson NP (2020) Multiple sclerosis and the risk of infection: considerations in the threat of the novel coronavirus, COVID-19/SARS-CoV-2. J Neurol 267(5):1567–1569. https://doi.org/10.1007/S00415-020-09822-3
Boziki MK, Mentis AFA, Shumilina M, Makshakov G, Evdoshenko E, Grigoriadis N (2020) COVID-19 immunopathology and the central nervous system: Implication for multiple sclerosis and other autoimmune diseases with associated demyelination. Brain Sci 10(6):1–11. https://doi.org/10.3390/brainsci10060345
Barzegar M, Vaheb S, Mirmosayyeb O, Afshari-Safavi A, Nehzat N, Shaygannejad V (2021) Can coronavirus disease 2019 (COVID-19) trigger exacerbation of multiple sclerosis? A retrospective study. Mult Scler Relat Disord 52:102947. https://doi.org/10.1016/J.MSARD.2021.102947
Buljevac D et al (2002) Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain 125(5):952–960. https://doi.org/10.1093/BRAIN/AWF098
Paules CI, Marston HD, Fauci AS (2020) Coronavirus Infections—More Than Just the Common Cold. JAMA 323(8):707–708. https://doi.org/10.1001/JAMA.2020.0757
Garjani A et al (2021) COVID-19 is associated with new symptoms of multiple sclerosis that are prevented by disease modifying therapies. Mult Scler Relat Disord 52:102939. https://doi.org/10.1016/J.MSARD.2021.102939
Yang J et al (2020) Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 94:91–95. https://doi.org/10.1016/J.IJID.2020.03.017
Di Stadio A, Romani L, Bernitsas E (2020, Nov) Could Sars-Cov2 affect MS progression? Mult Scler Relat Disord 46:102540. https://doi.org/10.1016/j.msard.2020.102540. Epub 2020 Sep 29. PMID: 33032060; PMCID: PMC7524432
Merad M, Martin JC (2020) Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 20(June):355–362. https://doi.org/10.1038/s41577-020-0331-4
Lindan CE et al (2021) Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Heal 5(3):167–177. https://doi.org/10.1016/S2352-4642(20)30362-X
Mehra B et al (2020) COVID-19-associated Severe Multisystem Inflammatory Syndrome in Children with Encephalopathy and Neuropathy in an Adolescent Girl with the Successful Outcome: An Unusual Presentation. Indian J Crit Care Med 24(12):1276. https://doi.org/10.5005/JP-JOURNALS-10071-23685
de Miranda Henriques-Souza AM, de Melo AC, de Aguiar Coelho Silva Madeiro B, Freitas LF, Sampaio Rocha-Filho PA, Gonçalves FG (2011) Acute disseminated encephalomyelitis in a COVID-19 pediatric patient. Neuroradiology 63(1):141–145. https://doi.org/10.1007/s00234-020-02571-0
McLendon LA, Rao CK, Da Hora CC, Islamovic F, Galan FN (2021) Post-COVID-19 acute disseminated encephalomyelitis in a 17-month-old. Pediatrics 147(6). https://doi.org/10.1542/peds.2020-049678
de Mol CL et al (2019) The clinical spectrum and incidence of anti-MOG-associated acquired demyelinating syndromes in children and adults. 26(7):806–814. https://doi.org/10.1177/1352458519845112
Khan A, Panwala H, Ramadoss D, Khubchandani R (2021) Myelin Oligodendrocyte Glycoprotein (MOG) Antibody Disease in a 11 Year Old with COVID-19 Infection. Indian J Pediatr 88(5):488–489. https://doi.org/10.1007/S12098-020-03656-7
Woodhall M, Mitchell JW, Gibbons E, Healy S, Waters P, Huda S (2020) Case Report: Myelin Oligodendrocyte Glycoprotein Antibody-Associated Relapse With COVID-19. Front Neurol 11:1479. https://doi.org/10.3389/FNEUR.2020.598531/BIBTEX
Apostolos-Pereira SL et al (2021) Clinical Features of COVID-19 on Patients With Neuromyelitis Optica Spectrum Disorders. Neurol Neuroimmunol Neuroinflammation 8(6):1060. https://doi.org/10.1212/NXI.0000000000001060
Zabalza A et al (2021) COVID-19 in multiple sclerosis patients: susceptibility, severity risk factors and serological response. Eur J Neurol 28(10):3384–3395. https://doi.org/10.1111/ene.14690
Parrotta et al (2020) COVID-19 outcomes in MS: Observational study of early experience from NYU Multiple Sclerosis Comprehensive Care Center. Neurol Neuroimmunol Neuroinflammation 7(5). https://doi.org/10.1212/NXI.0000000000000835
Klineova S et al (2021) Outcomes of COVID-19 infection in multiple sclerosis and related conditions: One-year pandemic experience of the multicenter New York COVID-19 Neuroimmunology Consortium (NYCNIC). Mult Scler Relat Disord 55(May):103153. https://doi.org/10.1016/j.msard.2021.103153
Stastna D et al (2021) Multiple sclerosis, neuromyelitis optica spectrum disorder and COVID-19: A pandemic year in Czechia. Mult Scler Relat Disord 54(May):103104. https://doi.org/10.1016/j.msard.2021.103104
Attauabi M et al (2020) Prevalence and Outcomes of COVID-19 Among Patients With Inflammatory Bowel Disease — A Danish Prospective Population-based Cohort Study. pp 1–11. https://doi.org/10.1093/ecco-jcc/jjaa205
Ashton JJ, Batra A, Coelho TA, Afzal NA, Beattie RM (2020) Challenges in chronic paediatric disease during the COVID-19 pandemic: diagnosis and management of inflammatory bowel disease in children. 105(7):321244. https://doi.org/10.1136/gutjnl-2020-321244
Neurath MF (2020) COVID-19 and immunomodulation in IBD. pp 1335–1342. https://doi.org/10.1136/gutjnl-2020-321269
Jablaoui A et al (2020) Fecal Serine Protease Profiling in Inflammatory Bowel Diseases (vol. 10, no. February). https://doi.org/10.3389/fcimb.2020.00021
Garg M et al (2015) Upregulation of circulating components of the alternative renin-angiotensin system in inflammatory bowel disease: A pilot study. https://doi.org/10.1177/1470320314521086
Kim KO, Jang BI (2022) Management of inflammatory bowel disease in the COVID-19 era (vol. 20, no. 1, pp. 3–10)
Miller DC et al (2020) Corticosteroid Injections and COVID-19 Infection Risk (vol. 21, no. July, pp. 1703–1706). https://doi.org/10.1093/pm/pnaa199
Danese S (2020) Management of IBD during the. Nat Rev Gastroenterol Hepatol. https://doi.org/10.1038/s41575-020-0294-8
D’amico F, Danese S, Peyrin-Biroulet L (2020) Systematic Review on Inflammatory Bowel Disease Patients With Coronavirus Disease 2019: It Is Time to Take Stock (no. January)
Current Summary Data | SECURE-IBD Database. https://covidibd.org/current-data/. Accessed 19 Oct 2022
Carparelli S, Pastore MR, Valvano MR, Marseglia A (2021) Worse impact of second wave COVID-19 pandemic in adults but not in children with inflammatory bowel disease: an Italian single tertiary center experience (pp 2744–2747)
Brenner EJ, Pigneur B, Focht G, Zhang X, Ungaro RC, Colombel JF, Turner D, Kappelman MD, Ruemmele FM (2020) Benign Evolution of SARS-Cov2 Infections in Children With Inflammatory Bowel Disease: Results From Two International (no. January)
Ricciuto A et al (2022) Inflammatory Bowel Disease Clinical Activity is Associated with COVID-19 Severity Especially in Younger Patients (pp 591–600)
Krawiec P, Opoka-Winiarska V, Pac-Kożuchowska E (2022, May 13) Is it inflammatory bowel disease flare or pediatric inflammatory multisystem syndrome temporally associated with COVID-19? J Clin Med 11(10):2765. https://doi.org/10.3390/jcm11102765
Nguyen H et al (2021) High Seroconversion Rate Against Severe Acute Respiratory Syndrome Coronavirus 2 in Symptomatic Pediatric Inflammatory Bowel Disease Patients (vol. 73, no. 3, pp. 363–366). https://doi.org/10.1097/MPG.0000000000003211
Bosa L et al (2022) Protective SARS-CoV-2 Antibody Response in Children With Inflammatory Bowel Disease Study Design and Population (vol. 10, no. February, pp. 1–9). https://doi.org/10.3389/fped.2022.815857
Grainger R, Machado PM, Robinson PC (2020) Novel coronavirus disease-2019 (COVID-19) in people with rheumatic disease: Epidemiology and outcomes (no. January)
Aloi M et al (202) Corona Virus Disease 2019 and Paediatric Inflammatory Bowel Diseases: Global Experience and Provisional Guidance ( March 2020 ) from the Paediatric IBD Porto Group of European Society of Paediatric (vol. 70, no. 6, pp 727–733). https://doi.org/10.1097/MPG.0000000000002729
Table S, March F, Iii I (2020) Clinical and Psychological Issues in Children with Inflammatory Bowel Disease During COVID-19 Pandemic (vol. XX, no. Xx, pp 1–2). https://doi.org/10.1093/ibd/izaa136
Trovato CM, Montuori M, Pietropaoli N, Oliva S (2021) COVID-19 and celiac disease: A pathogenetic hypothesis for a celiac outbreak. Int J Clin Pract 75(9):8–10. https://doi.org/10.1111/ijcp.14452
Uzzan M, Corcos O, Martin JC, Treton X, Bouhnik Y (2020) Why is SARS-CoV-2 infection more severe in obese men? The gut lymphatics – Lung axis hypothesis. Med Hypotheses 144(June):110023. https://doi.org/10.1016/j.mehy.2020.110023
Watts T et al (2005) Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Natl Acad Sci USA 102(8):2916–2921. https://doi.org/10.1073/pnas.0500178102
Jabri B, Sollid LM (2009, Dec) Tissue-mediated control of immunopathology in coeliac disease. Nat Rev Immunol 9(12):858–870. https://doi.org/10.1038/nri2670
Gholam-Mostafaei FS, Yadegar A, Aghdaei HA, Azimirad MMR, Zali I (2020) Gastroenterology and Liver Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, S. articles by ’Mohammad R. Zali’, and Z. MR, Anti-TNF containing regimens may be associated with increased risk of Clostridioides difficile infection in patients with underlying inflammatory bowel disease. Curr Res Transl Med
Zhen J et al (2021) The Risk of Contracting COVID-19 Is Not Increased in Patients With Celiac Disease. Clin Gastroenterol Hepatol 19(2):391–393. https://doi.org/10.1016/j.cgh.2020.10.009
Lionetti E, Fabbrizi A, Catassi C (2021, May) Prevalence of COVID-19 in Italian children with celiac disease: a cross-sectional study. Clin Gastroenterol Hepatol 19(5):1075. https://doi.org/10.1016/j.cgh.2020.11.035
Trovato CM, Montuori M, Cucchiara S, Oliva S (2020) ESPGHAN ‘biopsy-sparing’ guidelines for celiac disease in children with low antitransglutaminase during COVID-19. Eur J Gastroenterol Hepatol pp 1523–1526. https://doi.org/10.1097/MEG.0000000000001924
Catassi GN, Vallorani M, Cerioni F, Lionetti E, Catassi C (2020) A negative fallout of COVID-19 lockdown in Italy: Life-threatening delay in the diagnosis of celiac disease Dear (no. January)
Valitutti F, Troncone R, Pisano P, Ciacci C (2021) Where have all the other coeliacs gone in 2020? Road for a 2021 catch-up with missed diagnoses. Dig Liver Dis 53(4)
Asri N et al (2021) Toward finding the difference between untreated celiac disease and COVID-19 infected patients in terms of CD4, CD25 (IL-2 Rα), FOXP3 and IL-6 expressions as genes affecting immune homeostasis. BMC Gastroenterol 21(1). https://doi.org/10.1186/s12876-021-02056-1
Uche-Anya E, Husby S, Kaplan GG, Underwood FE, Green PHR, Lebwohl B (2021) An International Reporting Registry of Patients With Celiac Disease and COVID-19: Initial Results From SECURE-CELIAC. Clin Gastroenterol Hepatol 19(11):2435-2437.e4. https://doi.org/10.1016/j.cgh.2021.06.016
Lebwohl B, Söderling J, Roelstraete B, Lebwohl MG, Green PHR, Ludvigsson JF (2021) Risk of Severe Covid-19 in Patients with Celiac Disease: A Population-Based Cohort Study. J Am Acad Dermatol 85(6):1456–1464. https://doi.org/10.1016/j.jaad.2020.10.079
Cholankeril G et al (2020) Association of Digestive Symptoms and Hospitalization in Patients with SARS-CoV-2 Infection. Am J Gastroenterol 115(7):1129–1132. https://doi.org/10.14309/ajg.0000000000000712
Pan L et al (2020) Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: A descriptive, cross-sectional, multicenter study. Am J Gastroenterol 115(5):766–773. https://doi.org/10.14309/ajg.0000000000000620
Armstrong AW, Read C (2020) Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 323(19):1945–1960. https://doi.org/10.1001/JAMA.2020.4006
Telfer NR, Chalmers RJG, Whale K, Colman G (1992) The Role of Streptococcal Infection in the Initiation of Guttate Psoriasis. Arch Dermatol 128(1):39–42. https://doi.org/10.1001/ARCHDERM.1992.01680110049004
Naldi L (2022) Psoriasis Study Group of the Italian group for Epidemiological Research in Dermatology. Family history of psoriasis, stressful life events and recent infectious disease are risk factors for a first episode of acute guttate psoriasis: result of a case-control study. J Am Acad Dermatol 44:433–438. Available: https://cir.nii.ac.jp/crid/1572543026121687936. Accessed 19 Oct 2022
Seetharam KA, Sridevi L, Vidyasagar P (2011) Cutaneous manifestations of chikungunya fever. Indian Pediatr 49(1):51–53. https://doi.org/10.1007/S13312-012-0007-7
Beytout Q et al (2021) Impact of the COVID-19 pandemic on children with psoriasis. Ann Dermatol Venereol 148(2):106–111. https://doi.org/10.1016/J.ANNDER.2021.01.005
Bozonnat A, Assan F, LeGoff J, Bourrat E, Bachelez H (2021) SARS-CoV-2 infection inducing severe flare up of Deficiency of Interleukin Thirty-six (IL-36) Receptor Antagonist (DITRA) resulting from a mutation invalidating the activating cleavage site of the IL-36 receptor antagonist. J Clin Immunol 41(7):1511–1514. https://doi.org/10.1007/S10875-021-01076-6/FIGURES/1
Dermatologia N, Dermatology OUR (2013) Supplement 3 / 2013 (vol. 4, no. November, pp 583–665). https://doi.org/10.7241/ourd
Bordon J et al (2013) Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. Int J Infect Dis 17(2):e76–e83. https://doi.org/10.1016/j.ijid.2012.06.006
Khader S, Das S (2017) Yin and yang of interleukin-17 in host immunity to infection. F1000Research 6(MAY):1–11. https://doi.org/10.12688/f1000research.10862.1
Bosmann M, Ward PA (2012) Therapeutic potential of targeting IL-17 and IL-23 in sepsis. Clin Transl Med 1(1):3–7. https://doi.org/10.1186/2001-1326-1-4
Yiu ZZN et al (2021) Risk of hospitalization and death due to infection in people with psoriasis: a population-based cohort study using the Clinical Practice Research Datalink*. Br J Dermatol 184(1):78–86. https://doi.org/10.1111/bjd.19052
Wakkee M, Vries E, den Haak P, Tamar N (2011) Increased risk of infectious disease requiring hospitalization among patients with psoriasis: A population-based cohort. J Am Acad Dermatol 65(6)
Takeshita J, Shin DB, Ogdie A, Gelfand JM (2018) Risk of Serious Infection, Opportunistic Infection, and Herpes Zoster among Patients with Psoriasis in the United Kingdom. J Invest Dermatol 138(8):1726–1735. https://doi.org/10.1016/j.jid.2018.01.039
Georgakopoulos JR, Yeung J (2020) Patient-Driven Discontinuation of Dupilumab During the COVID-19 Pandemic in Two Academic Hospital Clinics at the University of Toronto. J Cutan Med Surg 24(4):422–423. https://doi.org/10.1177/1203475420930223
Yalcin AD, Yalcin AN (2021) Future perspective: biologic agents in patients with severe COVID-19. Immunopharmacol Immunotoxicol 43(1):1–7. https://doi.org/10.1080/08923973.2020.1818770
Fougerousse AC et al (2020) Systemic or biologic treatment in psoriasis patients does not increase the risk of a severe form of COVID-19. J Eur Acad Dermatol Venereol 34(11):e676–e679. https://doi.org/10.1111/jdv.16761
Olisova OY, Anpilogova EM, Svistunova DA (2020) Apremilast as a potential treatment option for COVID-19: No symptoms of infection in a psoriatic patient. Dermatol Ther 33(4). https://doi.org/10.1111/dth.13668
Queiro Silva R, Armesto S, González Vela C, Naharro Fernández C, González‐Gay MA (2020) COVID-19 patients with psoriasis and psoriatic arthritis on biologic immunosuppressant therapy vs apremilast in North Spain. Dermatol Ther 33(6). https://doi.org/10.1111/dth.13961
Mahil SK et al (200) Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19 . The COVID-19 resource centre is hosted on Elsevier Connect, the company’s public news and information (no. January)
Moja AJBMGCL (2016) Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf 15(sup1)
Zitouni J, Bursztejn A-C, Belloni Fortina A, Beauchet A, Di Lernia V, Lesiak A, Thomas J, Topkarci Z, Murashkin N, Brzezinski P, Torres T, Chiriac A, Luca C, McPherson T, Akinde M, Maruani A, Epishev R, Vidaurri de la Cruz H, Luna PC, Amy de la Bretêque M, Lasek A, Bourrat E, Bachelerie M, Mallet S, Steff M, Bellissen A, Neri I, Zafiriou E, van den Reek JMPA, Sonkoly E, Mahil SK, Smith CH, Flohr C, Bachelez H, Mahé E (2022) Children with psoriasis and COVID-19: factors associated with an unfavourable COVID-19 course, and the impact of infection on disease progression (Chi-PsoCov registry). J Eur Acad Dermatol Venereol 36:2076–2086. https://doi.org/10.1111/jdv.18361
Penso L, Dray-Spira R, Weill A, Zureik M, Sbidian E (2022, Jan) Psoriasis-related treatment exposure and hospitalization or in-hospital mortality due to COVID-19 during the first and second wave of the pandemic: Cohort study of 1 326 312 patients in France. Br J Dermatol 186(1):59–68. https://doi.org/10.1111/bjd.20659
Hamidi Z, Jabraeili-Siahroud S, Taati-Alamdari Y, Aghbash PS, Shamekh A, Baghi HB (2023) A comprehensive review of COVID-19 symptoms and treatments in the setting of autoimmune diseases. Virol J 20(1):1–11. https://doi.org/10.1186/S12985-023-01967-7/TABLES/1
Boddu SK, Aurangabadkar G, Kuchay MS (2020) New onset diabetes, type 1 diabetes and COVID-19. Diabetes Metab Syndr Clin Res Rev 14(6):2211–2217. https://doi.org/10.1016/J.DSX.2020.11.012
Paengsai N et al (2019) Recommended First-Line Antiretroviral Therapy Regimens and Risk of Diabetes Mellitus in HIV-Infected Adults in Resource-Limited Settings. Open Forum Infect Dis 6(10). https://doi.org/10.1093/OFID/OFZ298
Weinreich DM et al (2021) Regen-cov Antibody Combination and Outcomes in Outpatients with Covid-19. N Engl J Med 385(23):e81. https://doi.org/10.1056/NEJMOA2108163/SUPPL_FILE/NEJMOA2108163_DATA-SHARING.PDF
Gottlieb RL et al (2022) Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med 386(4):305–315. https://doi.org/10.1056/NEJMOA2116846
Hammond J et al (2022) Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med 386(15):1397–1408. https://doi.org/10.1056/NEJMOA2118542
Jayk Bernal A (2022) Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med 386(6):509–520. https://doi.org/10.1056/NEJMOA2116044
Qian G, Wang X, Patel NJ, Kawano Y, Fu X, Cook CE, Vanni KMM, Kowalski EN, Banasiak EP, Bade KJ, Srivatsan S, Williams ZK, Todd DJ, Weinblatt ME, Wallace ZS, Sparks JA (2023, Mar) Outcomes with and without outpatient SARS-CoV-2 treatment for patients with COVID-19 and systemic autoimmune rheumatic diseases: a retrospective cohort study. Lancet Rheumatol 5(3):e139–e150. https://doi.org/10.1016/S2665-9913(23)00006-1
Gerolymatou N et al (2022) Oral antiviral treatment for COVID-19 in patients with systemic autoimmune rheumatic diseases. jrheum.org, https://doi.org/10.3899/jrheum.221014
Nissen CB et al (2021) The role of antirheumatics in patients with COVID-19. Lancet Rheumatol 3(6):e447–e459. https://doi.org/10.1016/S2665-9913(21)00062-X
Wang M et al (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30(3):269–271. https://doi.org/10.1038/s41422-020-0282-0
Vincent MJ et al (2005) Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2. https://doi.org/10.1186/1743-422X-2-69
Mantovani Cardoso E, Hundal J, Feterman D, Magaldi J (2020) Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence? A case report and review of intertwining pathophysiology. Clin Rheumatol 39(9):2811–2815. https://doi.org/10.1007/S10067-020-05310-1
Eger K et al (2021) Poor outcome of SARS-CoV-2 infection in patients with severe asthma on biologic therapy. Respir Med 177(December 2020):106287. https://doi.org/10.1016/j.rmed.2020.106287
MacKenna B et al (2022) Risk of severe COVID-19 outcomes associated with immune-mediated inflammatory diseases and immune-modifying therapies: a nationwide cohort study in the OpenSAFELY platform. Lancet Rheumatol 4(7):e490–e506. https://doi.org/10.1016/S2665-9913(22)00098-4
Nikolopoulou GB, Maltezou HC (2022) COVID-19 in Children: Where do we Stand? Arch Med Res 53(1):1–8. https://doi.org/10.1016/J.ARCMED.2021.07.002
Zimmermann P, Pittet LF, Finn A, Pollard AJ, Curtis N (2022) Should children be vaccinated against COVID-19? Arch Dis Child 107(3):e1. https://doi.org/10.1136/ARCHDISCHILD-2021-323040
Acknowledgements
Parts of the figures were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/). Inkscape 1. 2. 1 was administered to create figures.
Author information
Authors and Affiliations
Contributions
P.S. and PS.P. wrote the main manuscript text and P.S. prepared figures. N.R. and PS.P. revised the manuscript critically for important intellectual content. All authors reviewed the manuscript and approved the version to be published.
Corresponding author
Ethics declarations
Ethical approval
Not applicable. The manuscript does not contain clinical studies or patient data.
Competing interests
The authors have no relevant financial or non-financial interests to disclose. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Communicated by Tobias Tenenbaum.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sadeghi, P., Pezeshki, P.S. & Rezaei, N. Coronavirus disease 2019 (COVID-19) in pediatric patients with autoimmune disorders. Eur J Pediatr 182, 2967–2988 (2023). https://doi.org/10.1007/s00431-023-04958-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-04958-6