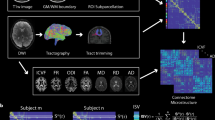
Abstract
The study aimed to investigate the consistency and diversity between metabolic and structural brain networks at individual level constructed with divergence-based method in healthy Chinese population. The 18F-FDG PET and T1-weighted images of brain were collected from 209 healthy participants. The Jensen-Shannon divergence (JSD) was used to calculate metabolic or structural connectivities between any pair of brain regions and then individual brain networks were constructed. The global and regional topological properties of both networks were analyzed with graph theoretical analysis. Regional properties including nodal efficiency, degree, and betweenness centrality were used to define hub regions of networks. Cross-modality similarity of brain connectivity was analyzed with differential power (DP) analysis. The default mode network (DMN) had the largest number of brain connectivities with high DP values. The small-worldness indexes of metabolic and structural networks in all participants were greater than 1. The structural network showed higher assortativity and local efficiency than metabolic network, while hierarchy and global efficiency were greater in the metabolic network (all P < 0.001). Most of hubs in both networks were symmetrically spatial distributed in the regions of the DMN and subcortical nuclei including thalamus and amygdala, etc. The human brain presented small-world architecture both in perspective of individual metabolic and structural networks. There was a structural substrate that supported the brain to globally and efficiently integrate and process metabolic interaction across brain regions. The cross-modality cooperation or specialization in both networks might imply mechanisms of achieving higher-order brain functions.





Similar content being viewed by others
Data availability
Jian-Guang Xu is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All data supporting our findings are available on reasonable request.
References
Achard S, Bullmore E (2007) Efficiency and cost of economical brain functional networks. PLoS Comput Biol 3(2):e17
Anderson MC, Floresco SB (2022) Prefrontal-hippocampal interactions supporting the extinction of emotional memories: the retrieval stopping model. Neuropsychopharmacology 47(1):180–195
Arnemann KL, Stöber F, Narayan S, Rabinovici GD, Jagust WJ (2017) Metabolic brain networks in aging and preclinical Alzheimer’s disease. NeuroImage Clin 17:987–999
Ashburner J, Friston KJ (2009) Computing average shaped tissue probability templates. Neuroimage 45(2):333–341
Bassett DS, Bullmore E (2006) Small-world brain networks. Neuroscientist 12(6):512–523
Batista-García-Ramó K, Fernández-Verdecia CI (2018) What we know about the brain structure-function relationship. Behav Sci (basel) 8(4):39
Bullmore ET, Bassett DS (2011) Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol 7:113–140
Bullmore E, Sporns O (2009) Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10(3):186–198
Cai B, Zhang G, Zhang A, Xiao L, Hu W, Stephen JM et al (2021) Functional connectome fingerprinting: Identifying individuals and predicting cognitive functions via autoencoder. Hum Brain Mapp 42(9):2691–2705
Choi YH, Yun JY, Kim BH, Lee MH, Song SK, Lee JM (2020) Gender-Related and hemispheric effects in cortical thickness-based hemispheric brain morphological network. Biomed Res Int 2020:3560259
Cole MW, Pathak S, Schneider W (2010) Identifying the brain’s most globally connected regions. Neuroimage 49(4):3132–3148
Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L et al (2016) The human brainnetome atlas : a new brain atlas based on connectional architecture. Cereb Cortex 26(8):3508–3526
Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM et al (2015) Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci 18(11):1664–1671
Fischl B (2012) Freesurfer. Neuroimage 62(2):774–781
Fischl B, Sereno MI, Dale AM (1999) Cortical surface-based analysis. I Segment Surf Reconstruc Neuroimage 9(2):195–207
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C et al (2002) Whole brain segmentation. Neuron 33(3):341–355
Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH et al (2004) Automatically parcellating the human cerebral cortex. Cereb Cortex 14(1):11–22
Gagnepain P, Hulbert J, Anderson MC (2017) Parallel regulation of memory and emotion supports the suppression of intrusive memories. J Neurosci 37(27):6423–6441
Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA et al (2015) An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489(7416):391–399
Hoge RD, Pike GB (2001) Oxidative metabolism and the detection of neuronal activation via imaging. J Chem Neuroanat 22(1–2):43–52
Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R et al (2009) Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci USA 106(6):2035–2040
Horn A, Ostwald D, Reisert M, Blankenburg F (2014) The structural-functional connectome and the default mode network of the human brain. Neuroimage 102:142–151
Imai M, Tanaka M, Sakata M, Wagatsuma K, Tago T, Toyohara J et al (2020) Metabolic network topology of alzheimer’s disease and dementia with lewy bodies generated using fluorodeoxyglucose positron emission tomography. J Alzheimer’s Dis 73(1):197–207
Inflation II, System SC, Fischl B, Sereno MI, Dale AM (1999) Cortical surface-based. Analysis 207:195–207
Jiang J, Zhou H, Duan H, Liu X, Zuo C, Huang Z et al (2017) A novel individual-level morphological brain networks constructing method and its evaluation in PET and MR images. Heliyon 3(12):e00475
Khalsa S, Mayhew SD, Chechlacz M, Bagary M, Bagshaw AP (2014) The structural and functional connectivity of the posterior cingulate cortex: comparison between deterministic and probabilistic tractography for the investigation of structure-function relationships. Neuroimage 102:118–127
Kong XZ, Liu Z, Huang L, Wang X, Yang Z, Zhou G et al (2015) Mapping individual brain networks using statistical similarity in regional morphology from MRI. PLoS ONE 10(11):1–24
Li R, Yin S, Zhu X, Ren W, Yu J, Wang P et al (2017) Linking inter-individual variability in functional brain connectivity to cognitive ability in elderly individuals. Front Aging Neurosci 9:1–13
Li Y-L, Wu J-J, Ma J, Li S-S, Xue X, Wei D et al (2021) Brain structural changes in carpal tunnel syndrome patients: from the perspectives of structural connectivity and structural covariance network. Neurosurgery 89(6):978–986
Li Y, Wu J, Ma J, Li S, Xue X, Wei D et al (2022) Alteration of the individual metabolic network of the brain based on jensen-shannon divergence similarity estimation in the elderly with type 2 diabetes mellitus. Diabetes 71(5):894–905
Li W, Tang Y, Wang Z, Hu S, Gao X 2021 The recognition pattern of individual brain metabolic connectome for parkinson’s disease identification. arXiv. 1–37.
Lin J (1991) Divergence measures based on the shannon entropy. IEEE Trans Inf Theory 37(1):145–151
Liu J, Liao X, Xia M, He Y (2018) Chronnectome fingerprinting: Identifying individuals and predicting higher cognitive functions using dynamic brain connectivity patterns. Hum Brain Mapp 39(2):902–915
Logothetis NK (2008) What we can do and what we cannot do with fMRI. Nature 453(7197):869–878
Lu Y-C, Wu J-J, Ma H, Hua X-Y, Xu J-G (2020) Functional organization of brain network in peripheral neural anastomosis rats after electroacupuncture: an ICA and connectome analysis. Neuroscience 442:216–227
Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, Langs G et al (2016) Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci USA 113(44):12574–12579
Menon SS, Krishnamurthy K (2019) A comparison of static and dynamic functional connectivities for identifying subjects and biological sex using intrinsic individual brain connectivity. Sci Rep 9(1):1–11
Misciagna S (2013) Positron emission tomography-recent developments in instrumentation, research and clinical oncological practice. IntechOpen, Rijeka
Moon Y, Rajagopalan B, Lall U (1995) Estimation of mutual information using kernel density estimators. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Top 52(3):2318–2321
Mueller S, Wang D, Fox MD, Yeo BT, Sepulcre J, Sabuncu MR et al (2013) Individual variability in functional connectivity architecture of the human brain. Neuron 77(3):586–595
Murphy KP 2012 Machine learning: a probabilistic perspective. Murphy KP (ed). The MIT Press. Cambridge, USA. 56–61.
Nielsen F (2020) On a generalization of the jensen-shannon divergence and the jensen-shannon centroid. Entropy 22(2):221
Raichle ME (2015) The brain’s default mode network. Annu Rev Neurosci 38:433–447
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci U S A 98(2):676–682
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52(3):1059–1069
Salvador R, Suckling J, Coleman MR, Pickard JD, Menon D, Bullmore E (2005) Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex 15(9):1332–2342
Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME et al (1997) Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci 9(5):648–663
Tewarie P, Hillebrand A, van Dellen E, Schoonheim MM, Barkhof F, Polman CH et al (2014) Structural degree predicts functional network connectivity: a multimodal resting-state fMRI and MEG study. Neuroimage 97:296–307
Thomas Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M et al (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106(3):1125–1165
Trusina A, Maslov S, Minnhagen P, Sneppen K (2004) Hierarchy measures in complex networks. Phys Rev Lett 92(17):1–4
van den Heuvel MP, Sporns O (2013) Network hubs in the human brain. Trends Cogn Sci 17(12):683–696
Vatansever D, Menon DK, Manktelow AE, Sahakian BJ, Stamatakis EA (2015) Default mode dynamics for global functional integration. J Neurosci 35(46):15254–15262
Venkatesh M, Jaja J, Pessoa L (2020) Comparing functional connectivity matrices: a geometry-aware approach applied to participant identification. Neuroimage 207:116398
Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME et al (2006) Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol 96(6):3517–3531
Wang J, Wang X, Xia M, Liao X, Evans A, He Y (2015a) GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci 9:1–16
Wang Z, Dai Z, Gong G, Zhou C, He Y (2015b) Understanding structural-functional relationships in the human brain: a large-scale network perspective. Neuroscientist 21(3):290–305
Wang H, Jin X, Zhang Y, Wang J (2016) Single-subject morphological brain networks: connectivity mapping, topological characterization and test-retest reliability. Brain Behav 6(4):1–21
Wang M, Jiang J, Yan Z, Alberts I, Ge J, Zhang H et al (2020) Individual brain metabolic connectome indicator based on Kullback-Leibler Divergence Similarity Estimation predicts progression from mild cognitive impairment to Alzheimer’s dementia. Eur J Nucl Med Mol Imaging 47(12):2753–2764
Wang H, Fan L, Song M, Liu B, Wu D, Jiang R et al (2021a) Functional connectivity predicts individual development of inhibitory control during adolescence. Cereb Cortex 31(5):2686–2700
Wang W-L, Li Y-L, Zheng M-X, Hua X-Y, Wu J-J, Yang F-F et al (2021b) Altered topological properties of grey matter structural covariance networks in complete thoracic spinal cord injury patients: a graph theoretical network analysis. Neural Plast 2021:8815144
Watts DJ, Strogatz SH (1998) Collective dynamics of 'small world' networks. Nature 393(6684):440–442
Wu J, Wang S, Lu Y, Zheng M, Hua X, Xu J (2020) Shifted hub regions in the brain network of rat neuropathic pain model after electroacupuncture therapy. J Integr Neurosci 19(1):65–75
Xu P, Huang R, Wang J, Van Dam NT, Xie T, Dong Z et al (2014) Different topological organization of human brain functional networks with eyes open versus eyes closed. Neuroimage 90:246–255
Yakushev I, Drzezga A, Habeck C (2017) Metabolic connectivity: methods and applications. Curr Opin Neurol 30(6):677–685
Yakushev I, Ripp I, Wang M, Savio A, Schutte M, Lizarraga A et al (2022) Mapping covariance in brain FDG uptake to structural connectivity. Eur J Nucl Med Mol Imaging 49(4):1288–1297
Zhen KX, Wang X, Huang L, Pu Y, Yang Z, Dang X et al (2014) Measuring individual morphological relationship of cortical regions. J Neurosci Methods 237:103–107
Acknowledgements
This work was supported by the National Key R&D Program of China [Grant Nos.: 2018YFC2001600, and 2018YFC2001604]; National Natural Science Foundation of China [Grant Nos.: 81802249, 81871836, 81874035, and 81902301]; Shanghai Science and Technology Committee [Grant Nos.: 18511108300, 18441903900, and 18441903800]; Shanghai Rising-Star Program [Grant No.: 19QA1409000]; Shanghai Municipal Commission of Health and Family Planning [Grant No.: 2018YQ02, and 201840224]; Shanghai Youth Top Talent Development Plan and Shanghai “Rising Stars of Medical Talent” Youth Development Program [Grant No.: RY411.19.01.10]; Program of Shanghai Academic Research Leader [Grant No.: 19XD1403600].
Funding
This work was supported by the National Key R&D Program of China (Grant Nos.: 2018YFC2001600, and 2018YFC2001604); National Natural Science Foundation of China (Grant Nos.: 81802249, 81871836, 81874035, and 81902301); Shanghai Science and Technology Committee (Grant Nos.: 18511108300, 18441903900, and 18441903800); Shanghai Rising-Star Program (Grant No.: 19QA1409000); Shanghai Municipal Commission of Health and Family Planning (Grant No.: 2018YQ02, and 201840224); Shanghai Youth Top Talent Development Plan and Shanghai “Rising Stars of Medical Talent” Youth Development Program (Grant No.: RY411.19.01.10); Program of Shanghai Academic Research Leader (Grant No.: 19XD1403600).
Author information
Authors and Affiliations
Contributions
Conceptualization: X-YH, M-XZ, J-JW; Methodology: X-YH, M-XZ, J-JW; Validation: CLS, X-G, J-PZ, DW; Formal Analysis: X-YH, M-XZ, J-JW, Y-LL; Writing-Original Draft: Y-LL, M-XZ; Writing Review & Editing: J-GX; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was approved by the Ethics Committee of the Yueyang Hospital of Integrated Traditional Chinese and Western Medicine affiliated to Shanghai University of Traditional Chinese Medicine.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, YL., Zheng, MX., Hua, XY. et al. Cross-modality comparison between structural and metabolic networks in individual brain based on the Jensen-Shannon divergence method: a healthy Chinese population study. Brain Struct Funct 228, 761–773 (2023). https://doi.org/10.1007/s00429-023-02616-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-023-02616-z