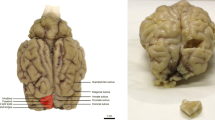
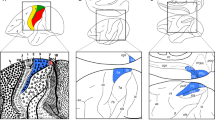
Abstract
The architecture of the neocortex classically consists of six layers, based on cytological criteria and on the layout of intra/interlaminar connections. Yet, the comparison of cortical cytoarchitectonic features across different species proves overwhelmingly difficult, due to the lack of a reliable model to analyze the connection patterns of neuronal ensembles forming the different layers. We first defined a set of suitable morphometric cell features, obtained in digitized Nissl-stained sections of the motor cortex of the horse, chimpanzee, and crab-eating macaque. We then modeled them using a quite general non-parametric data representation model, showing that the assessment of neuronal cell complexity (i.e., how a given cell differs from its neighbors) can be performed using a suitable measure of statistical dispersion such as the mean absolute deviation—mean absolute deviation (MAD). Along with the non-parametric combination and permutation methodology, application of MAD allowed not only to estimate, but also to compare and rank the motor cortical complexity across different species. As to the instances presented in this paper, we show that the pyramidal layers of the motor cortex of the horse are far more irregular than those of primates. This feature could be related to the different organizations of the motor system in monodactylous mammals.




Similar content being viewed by others
Change history
18 April 2017
An erratum to this article has been published.
Notes
“Member States should, where appropriate, facilitate the establishment of programmes for sharing the organs and tissue of animals that are killed.”
References
Aradi I, Soltesz I (2002) Modulation of network behaviour by changes in variance in interneuronal properties. J Physiol (Lond) 538:227–251. doi:10.1113/jphysiol.2001.013054
Arboretti GR, Bonnini S, Corain L, Salmaso L (2014) A Permutation approach for ranking of multivariate Populations. J Multivar Anal 132:39–57. doi:10.1016/j.jmva.2014.07.009
Barone R (1959) Observations sur le faisceau pyramidal des équidés. Bull Soc Sci Vet Med Comp Lyon 5:265–271
Barone R (1966) Observations sur le faisceau rubro-spinal des équidés. C.R. Assoc Anat 131:115–121
Barone R, Bortolami R (2004) Anatomie comparée des mammifères domestiques. 6/1 Système Nerveux Central. Vigot, Paris
Beul SF, Hilgetag CC (2015). Front Neuroanat 8:165. doi:10.3389/fnana.2014.00165
Bianchi S, Stimpson CD, Duka T, Larsen MD, Janssen WGM, Collins Z, Bauernfeind AL, Schapiro SJ, Baze WB, McArthur MJ, Hopkins WD, Wildman DE, Lipovich L, Kuzawa CW, Jacobs B, Hof PR, Sherwood CC (2013) Synaptogenesis and development of pyramidal neuron dendritic morphology in the chimpanzee neocortex resembles humans. Proc Natl Acad Sci USA 110(suppl 2):10395–10401. doi:10.1073/pnas.1301224110
Breazile JE, Swafford BC, Biles DR (1966) Motor cortex of the horse. Am J Vet Res 27:1605–1609
Breazile JE, Jennings DP, Swafford BC (1967) Conduction velocities in the corticospinal tract of the horse. Exp Neurol 17:357–363
Brodmann K (1909) Localisation in the cerebral cortex. Smith-Gordon, London
Brown AR, Teskey GC (2014) Motor cortex is functionally organized as a set of spatially distinct representations for complex movements. J Neurosci 34:13574–13585. doi:10.1523/jneurosci.2500-14.2014
Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI (2009) Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459:663–667. doi:10.1038/nature08002
Charvet CJ, Reep RL, Finlay BL (2016) Evolution of cytoarchitectural landscapes in the mammalian isocortex: Sirenians (Trichechus manatus) in comparison with other mammals. J Comp Neurol 524:772–782. doi:10.1002/cne.23864
Chiocchetti R, Bombardi C, Grandis A, Mazzuoli G, Gentile A, Pisoni L, Joechler M, Lucchi ML (2006) Cytoarchitecture, morphology and lumbosacral spinal cord projections of the cattle red nucleus. Am J Vet Res 67:10. doi:10.2460/avjr.67.10.1662
Constantinople CM, Bruno RM (2013) Deep cortical layers are activated directly by thalamus. Science 340:1591–1594. doi:10.1126/science.1236425
Corain L, Salmaso L (2004) Multivariate and multistrata nonparametric tests: the nonparametric combination method. J Mod App Stat Meth 3:443–461
Corain L, Salmaso L (2007) A nonparametric method for defining a global preference ranking of industrial products. J Appl Stat 34:203–216. doi:10.1080/02664760600995122
Corain L, Arboretti R, Bonnini S (2016) Ranking of multivariate populations—a permutation approach with applications. CRC press, Boca Raton
Cozzi B, Povinelli M, Ballarin C, Granato A (2014) The brain of the horse: weight and cephalization quotients. Brain Behav Evol 83:9–16. doi:10.1159/000356527
Delucchi MR, Dennis BJ, Adey WR (1965) A stereotaxic atlas of the chimpanzee brain (Pan satyrus). University of California Press, Berkeley
Ebina T, Sohya K, Imayoshi I, Yin ST, Kimura R, Yanagawa Y, Kameda H, Hioki H, Kaneko T, Tsumoto T (2014) 3D clustering of GABAergic neurons enhances inhibitory actions on excitatory neurons in the mouse visual cortex. Cell Rep 9:1896–1907. doi:10.1016/j.celrep.2014.10.057
Elston GN, Manger PR (2014) Pyramidal cells in V1 of African rodents are bigger, more branched and more spiny than those in primates. Front Neuroanat 8:4. doi:10.3389/fnana.2014.00004
Galarreta M, Hestrin S (1999) A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature 402:72–75. doi:10.1038/47029
García-Cabezas MA, Barbas H (2014) Area 4 has layer IV in adult primates. Eur J Neurosci 39:1824–1834. doi:10.1111/ejn.12585
Geyer S, Luppino G, Rozzi S (2011) Motor cortex. In: Mai JK, Paxinos G (eds) The human nervous system, 3rd edn. Academic, Amsterdam, pp 1012–1035
Gibson JR, Beierlein M, Connors BW (1999) Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402:75–79. doi:10.1038/47035
Gilman JP, Medalla M, Luebke JI (2016) Area-specific features of pyramidal neurons—a comparative study in mouse and Rhesus monkey. Cereb Cortex. doi:10.1093/cercor/bhw062
Godlove DC, Maier A, Woodman GF, Schall JD (2014) Microcircuitry of agranular frontal cortex: testing the generality of the canonical cortical microcircuit. J Neurosci 34:5355–5369. doi:10.1523/jneurosci.5127-13.2014
Grandis A, Bombardi C, Travostini B, Gentile A, Joechler M, Pisoni L, Chiocchetti R (2007) Vestibular nuclear complex in cattle: Topography, morphology, cytoarchitecture and lumbo-sacral projections. J Vestib Res 17:9–24
Haarmann K (1974) Morphologische und histologische untersuchungen am neocortex einiger Perissodactyla. Acta Anat (Basel) 90:285–299
Habib El Amir EA (2012) On uses of mean absolute deviation: decomposition, skewness and correlation coefficients. Metron 70:145–164
Heiffner R, Masterton B (1975) Variation in form of the pyramidal tract and its relationship to digital dexterity. Brain Behav Evol 12:161–200
Henssen A, Zilles K, Palomero-Gallagher N, Schleicher A, Mohlberg H, Gerboga F, Eickhoff SB, Bludau S, Amunts K (2016) Cytoarchitecture and probability maps of the human medial orbitofrontal cortex. Cortex 75:87–112. doi:10.1016/j.cortex.2015.11.006
Herculano-Houzel S, Manger P, Kaas JH (2014) Brain scaling in mammalian evolution as a consequence of concerted and mosaic changes in numbers of neurons and average cell size. Front Neuroanat 8:77. doi:10.3389/fnana.2014.00077
Hof PR, Glezer II, Condé F, Flagg RA, Rubin MB, Nimchinsky EA, Vogt Weisenhorn DM (1999) Cellular distribution of the calcium-binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: phylogenetic and developmental patterns. J Chem Neuroanat 16:77–116. doi:10.1016/S0891-0618(98)00065-9
Hof PR, Glezer II, Nimchinsky EA, Erwin JM (2000) Neurochemical and cellular specializations in the mammalian neocortex reflect phylogenetic relationships: evidence from primates, cetaceans, and artiodactyls. Brain Behav Evol 55:300–310
Johnson JI (1990) Comparative development of somatic sensory cortex. In: Jones EG, Peters A (eds) Cerebral Cortex, vol 8B. Comparative structure and evolution of cerebral cortex, part II. Plenum Press, New York, pp 335–449
Katz PS (2016) Evolution of central pattern generators and rhythmic behaviours. Philos Trans R Soc Lond B Biol Sci 371:20150057. doi:10.1098/rstb.2015.0057
Kazu RS, Maldonado J, Mota B, Manger PR, Herculano-Houzel S (2014) Cellular scaling rules for the brain of Artiodactyla include a highly folded cortex with few neurons. Front Neuroanat 8:128. doi:10.3389/fnana.2014.00128
Kazu RS, Maldonado J, Mota B, Manger PR, Herculano-Houzel S (2015) Corrigendum: Cellular scaling rules for the brain of Artiodactyla include a highly folded cortex with few neurons. Front Neuroanat 9:39. doi:10.3389/fnana.2015.00039
Luengo-Sanchez S, Bielza C, Benavides-Piccione R, Fernaud-Espinosa I, DeFelipe J, Larrañaga P (2015) A univocal definition of the neuronal soma morphology using Gaussian mixture models. Front Neuroanat 9:137. doi:10.3389/fnana.2015.00137
Martin del Campo H, Measor K, Razak KA (2014) Parvalbumin and Calbindin expression in parallel thalamocortical pathways in a gleaning bat, Antrozous pallidus. J Comp Neurol 522:2431–2445. doi:10.1002/cne.23541
McLachlan G, Peel D (2004) Finite mixture models. Wiley, New York
Montelli S, Suman M, Corain L, Cozzi B, Peruffo A (2016) Sexually diergic trophic effects of estradiol exposure on developing bovine cerebellar granule cells. Neuroendocrinology. doi:10.1159/000444528
Morgane PJ, Glezer II, Jacobs MS (1988) Visual cortex of the dolphin: an image analysis study. J Comp Neurol 273:3–25
Musienko PE, Deliagina TG, Gerasimenko YP, Orlovsky GN, Zelenin PV (2014) Limb and trunk mechanisms for balance control during locomotion in quadrupeds. J Neurosci 34:5704–5716. doi:10.1523/jneurosci.4663-13.2014
Nieuwenhuys R, ten Donkelaar HJ, Nicholson C (1998) The central nervous system of vertebrates, vol 3. Springer-Verlag, Heidelberg, p 1640
Orban GA (2016) Functional definitions of parietal areas in human and non-human primates. Proc Biol Sci 283:20160118. doi:10.1098/rspb.2016.0118
Paxinos G, Huang X-F, Toga AW (1999) The rhesus monkey brain in stereotaxic coordinates. Academic, San Diego
Pesarin F, Salmaso L (2010) Permutation tests for complex data-theory and software. Wiley, Chichester
Pham-Gia T, Hung TL (2001) The mean and median absolute deviations. Math Comput Model 34:921–936. doi:10.1016/S0895-7177(01)00109-1
Ramón y Cajal S (1917). Recuerdos de mi vida. Tomo II, Historia de mi labor científica. Imprenta y Librería de Nicolás Moya, Madrid
Schaffelhofer S, Agudelo-Toro A, Scherberger H (2015) Decoding a wide range of hand configurations from macaque motor, premotor, and parietal cortices. J Neurosci 35:1068–1081. doi:10.1523/jneurosci.3594-14.2015
Schleicher A, Amunts K, Geyer S, Kowalski T, Schormann T, Palomero-Gallagher N, Zilles K (2000) A stereological approach to human cortical architecture: identification and delineation of cortical areas. J Chem Neuroanat 20:31–47. doi:10.1016/S0891-0618(00)00076-4
Seiferle E (1975) Nervensystem. In: Nickel R, Schummer A, Seiferle E (eds) Lehrbuch der anatomie der haustiere, vol 4. Verlag Paul Parey, Berlin und Hamburg, pp 3–188
Towe AL (1973) Relative numbers of pyramidal tract neurons in mammals of different sizes. Brain Behav Evol 7:1–17
Verhaart WJC (1962) The pyramidal tract. Its structure and functions in man and animals. World Neurol 3:43–53
Zilles K, Palomero-Gallagher N, Amunts K (2013) Development of cortical folding during evolution and ontogeny. Trends Neurosci 36:275–284. doi:10.1016/j.tins.2013.01.006
Acknowledgements
The present study has been sponsored by Grants from the University of Padova to BC, AP, LC. The authors would like to thank Dr. Sandro Mazzariol for performing the post-mortem on the chimpanzees and horses, and Dr. Giuseppe Palmisano and Dr. Michele Povinelli for their help in sampling the tissues, all from the Dept. of Comparative Biomedicine and Food Science of the University of Padova. The Authors also wish to thank Maria Rosa Pittarello from the “Pietro Arduino” Library of the University of Padova at Legnaro (PD) for her precious help in tracing old literature.
Author information
Authors and Affiliations
Corresponding authors
Additional information
An erratum to this article is available at https://doi.org/10.1007/s00429-017-1397-z.
Rights and permissions
About this article
Cite this article
Cozzi, B., De Giorgio, A., Peruffo, A. et al. The laminar organization of the motor cortex in monodactylous mammals: a comparative assessment based on horse, chimpanzee, and macaque. Brain Struct Funct 222, 2743–2757 (2017). https://doi.org/10.1007/s00429-017-1369-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-017-1369-3