Abstract
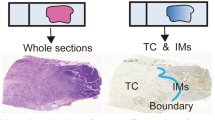
Accurate predictions on prognosis and neoadjuvant therapy response are crucial for esophagogastric junction adenocarcinoma (EGJA) patients. Therefore, we aimed to investigate the predictive abilities of several indicators, including tumor stroma ratio (TSR), tumor stroma maturity (TSM), and the density and spatial distribution of tumor-infiltrating immune cells (TIICs), such as T cells, B cells, and tumor-associated macrophages (TAMs). Resection and biopsy specimens of a total of 695 patients were included, obtained from the National Cancer Center (NCC) and The Cancer Genome Atlas (TCGA) cohorts. TSR and TSM were evaluated based on histological assessment. TIICs were quantified by QuPath following immunohistochemical (IHC) staining in resection specimens, while the Klintrup–Mäkinen (KM) grade was employed for evaluating TIIC in biopsy specimens. Patients with high stromal levels or immature stroma had relatively worse prognoses. Furthermore, high CD8+T cell count in the tumor periphery, as well as low CD68+ TAM count either in the tumor center or in the tumor periphery, was an independent favorable prognostic factor. Significantly, the combination model incorporating TSM and CD163+TAMs emerged as an independent prognostic factor in both two independent cohorts (HR 3.644, 95% CI 1.341–9.900, p = 0.011 and HR 1.891, 95% CI 1.195–2.99, p = 0.006, respectively). Additionally, high stromal levels in preoperative biopsies correlated with poor neoadjuvant therapy response (p < 0.05). In conclusion, our findings suggest that TSR, TSM, CD8+T cell, CD68+TAMs, and CD163+TAMs predict the prognosis to some extent in patients with EGJA. Notably, the combined model incorporating TSM and CD163+TAM can contribute significantly to prognostic stratification. Additionally, high stromal levels evaluated in preoperative biopsy specimens correlated with poor neoadjuvant therapy response.




Similar content being viewed by others
Data availability
1. The datasets used and/or analyzed in our cohort are available from the corresponding author on reasonable request.
2. The datasets used in our study from TCGA cohort are available in TCGA-STAD cohort (https://portal.gdc.cancer.gov/projects/TCGA-STAD).
Abbreviations
- CAFs:
-
Cancer-associated fibroblasts
- CPS:
-
Combined positive score
- EGJA:
-
Esophagogastric junction adenocarcinoma
- GMS:
-
Glasgow microenvironment score
- HE:
-
Hematoxylin and eosin
- IHC:
-
Immunohistochemical
- MPR:
-
Major pathological response
- pCR:
-
Pathologic complete regression
- TAMs:
-
Tumor-associated macrophages
- TCGA:
-
The Cancer Genome Atlas
- TIICs:
-
Tumor-infiltrating immune cells
- TME:
-
Tumor microenvironment
- TRG:
-
Tumor regression grade
- TSR:
-
Tumor stroma ratio
- TSM:
-
Tumor stroma maturity
- Tc:
-
Tumor center
- Tp:
-
Tumor periphery
References
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Chevallay M, Bollschweiler E, Chandramohan SM et al (2018) Cancer of the gastroesophageal junction: a diagnosis, classification, and management review. Ann N Y Acad Sci 1434:132–138
Thrift AP, Whiteman DC (2012) The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol: Off J Eur Soc Med Oncol 23:3155–3162
Matsuno K, Ishihara R, Ohmori M et al (2019) Time trends in the incidence of esophageal adenocarcinoma, gastric adenocarcinoma, and superficial esophagogastric junction adenocarcinoma. J Gastroenterol 54:784–791
Hatta W, Tong D, Lee YY et al (2017) Different time trend and management of esophagogastric junction adenocarcinoma in three Asian countries. Dig Endosc 29(Suppl 2):18–25
Liu K, Yang K, Zhang W et al (2016) Changes of esophagogastric junctional adenocarcinoma and gastroesophageal reflux disease among surgical patients during 1988–2012: a single-institution, high-volume experience in China. Ann Surg 263:88–95
Ajani JA, D’Amico TA, Bentrem DJ et al (2022) Gastric Cancer, Version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw: JNCCN 20:167–192
Alsina M, Arrazubi V, Diez M et al (2023) Current developments in gastric cancer: from molecular profiling to treatment strategy. Nat Rev Gastroenterol Hepatol 20:155–170
Shah MA, Kennedy EB, Alarcon-Rozas AE et al (2023) Immunotherapy and targeted therapy for advanced gastroesophageal cancer: ASCO guideline. J Clin Oncol 41:1470–1491
Wu T, Dai Y (2017) Tumor microenvironment and therapeutic response. Cancer Lett 387:61–68
Giraldo NA, Sanchez-Salas R, Peske JD et al (2019) The clinical role of the TME in solid cancer. Br J Cancer 120:45–53
Mao X, Xu J, Wang W et al (2021) Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer 20:131
Martinez-Outschoorn UE, Lisanti MP, Sotgia F (2014) Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol 25:47–60
Kobayashi H, Enomoto A, Woods SL et al (2019) Cancer-associated fibroblasts in gastrointestinal cancer. Nat Rev Gastroenterol Hepatol 16:282–295
Farhood B, Najafi M, Mortezaee K (2019) Cancer-associated fibroblasts: secretions, interactions, and therapy. J Cell Biochem 120:2791–2800
Chen Y, McAndrews KM, Kalluri R (2021) Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol 18:792–804
Vangangelt KMH, Green AR, Heemskerk IMF et al (2020) The prognostic value of the tumor-stroma ratio is most discriminative in patients with grade III or triple-negative breast cancer. Int J Cancer 146:2296–2304
Kramer CJH, Vangangelt KMH, van Pelt GW et al (2019) The prognostic value of tumour-stroma ratio in primary breast cancer with special attention to triple-negative tumours: a review. Breast Cancer Res Treat 173:55–64
Ueno H, Kanemitsu Y, Sekine S et al (2017) Desmoplastic pattern at the tumor front defines poor-prognosis subtypes of colorectal cancer. Am J Surg Pathol 41:1506–1512
Akimoto N, Väyrynen JP, Zhao M et al (2022) Desmoplastic reaction, immune cell response, and prognosis in colorectal cancer. Front Immunol 13:840198
Peng C, Liu J, Yang G et al (2018) The tumor-stromal ratio as a strong prognosticator for advanced gastric cancer patients: proposal of a new TSNM staging system. J Gastroenterol 53:606–617
Yim K, Jang WM, Cho U et al (2022) Intratumoral budding in pretreatment biopsies, among tumor microenvironmental components, can predict prognosis and neoadjuvant therapy response in colorectal adenocarcinoma. Medicina (Kaunas, Lithuania) 58
Liang Y, Zhu Y, Lin H et al (2021) The value of the tumour-stroma ratio for predicting neoadjuvant chemoradiotherapy response in locally advanced rectal cancer: a case control study. BMC Cancer 21:729
Wen X, Zee SY, Shroyer KR et al (2022) Intratumoral budding and tumor microenvironment in pretreatment rectal cancer biopsies predict the response to neoadjuvant chemoradiotherapy. Appl Immunohistochem Mol Morphol : AIMM 30:1–7
van Pelt GW, Krol JA, Lips IM et al (2020) The value of tumor-stroma ratio as predictor of pathologic response after neoadjuvant chemoradiotherapy in esophageal cancer. Clin Transl Radiat Oncol 20:39–44
Bruni D, Angell HK, Galon J (2020) The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer 20:662–680
Chen DS, Mellman I (2017) Elements of cancer immunity and the cancer-immune set point. Nature 541:321–330
Yu CC, Wortman JC, He TF et al (2021) Physics approaches to the spatial distribution of immune cells in tumors. Rep Progress Phys Phys Soc (Great Britain) 84:022601
Huang Q, Read M, Gold JS et al (2020) Unraveling the identity of gastric cardiac cancer. J Dig Dis 21:674–686
van Pelt GW, Kjær-Frifeldt S, van Krieken J et al (2018) Scoring the tumor-stroma ratio in colon cancer: procedure and recommendations. Virchows Archiv : Int J Pathol 473:405–412
Kemi N, Eskuri M, Kauppila JH (2019) Tumour-stroma ratio and 5-year mortality in gastric adenocarcinoma: a systematic review and meta-analysis. Sci Rep 9:16018
Kemi NA, Eskuri M, Pohjanen VM et al (2019) Histological assessment of stromal maturity as a prognostic factor in surgically treated gastric adenocarcinoma. Histopathology 75:882–889
Ueno H, Ishiguro M, Nakatani E et al (2021) Prognostic value of desmoplastic reaction characterisation in stage II colon cancer: prospective validation in a phase 3 study (SACURA Trial). Br J Cancer 124:1088–1097
Ueno H, Jones A, Jass JR et al (2002) Clinicopathological significance of the ‘keloid-like’ collagen and myxoid stroma in advanced rectal cancer. Histopathology 40:327–334
Janjigian YY, Shitara K, Moehler M et al (2021) First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (London, England) 398:27–40
Rha SY, Oh DY, Yañez P et al (2023) Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 24:1181–1195
Klintrup K, Mäkinen JM, Kauppila S et al (2005) Inflammation and prognosis in colorectal cancer. Eur J Cancer (Oxford, England: 1990) 41:2645–2654
Mandard AM, Dalibard F, Mandard JC et al (1994) Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic Correl Cancer 73:2680–2686
Koh YW, Park YS, Ryu MH et al (2013) Postoperative nodal status and diffuse-type histology are independent prognostic factors in resectable advanced gastric carcinomas after preoperative chemotherapy. Am J Surg Pathol 37:1022–1029
Li S, Yu W, Xie F et al (2023) Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat Commun 14:8
Fu M, Chen D, Luo F et al (2020) Association of the tumour stroma percentage in the preoperative biopsies with lymph node metastasis in colorectal cancer. Br J Cancer 122:388–396
Jing CY, Fu YP, Huang JL et al (2018) Prognostic nomogram based on histological characteristics of fibrotic tumor stroma in patients who underwent curative resection for intrahepatic cholangiocarcinoma. Oncologist 23:1482–1493
Ueno H, Kanemitsu Y, Sekine S et al (2019) A multicenter study of the prognostic value of desmoplastic reaction categorization in stage II colorectal cancer. Am J Surg Pathol 43:1015–1022
Rimal R, Desai P, Daware R et al (2022) Cancer-associated fibroblasts: origin, function, imaging, and therapeutic targeting. Adv Drug Deliv Rev 189:114504
Chen D, Chen H, Chi L et al (2021) Association of tumor-associated collagen signature with prognosis and adjuvant chemotherapy benefits in patients with gastric cancer. JAMA Netw Open 4:e2136388
Brett EA, Sauter MA, Machens HG et al (2020) Tumor-associated collagen signatures: pushing tumor boundaries. Cancer Metab 8:14
Wernicke M, Piñeiro LC, Caramutti D et al (2003) Breast cancer stromal myxoid changes are associated with tumor invasion and metastasis: a central role for hyaluronan. Mod Pathol 16:99–107
Zheng X, Jiang K, Xiao W et al (2022) CD8(+) T cell/cancer-associated fibroblast ratio stratifies prognostic and predictive responses to immunotherapy across multiple cancer types. Front Immunol 13:974265
Xu L, Zhong W, Li C et al (2023) The tumour-associated stroma correlates with poor clinical outcomes and immunoevasive contexture in patients with upper tract urothelial carcinoma: results from a multicenter real-world study (TSU-01 study). Br J Cancer 128:310–320
Maller O, Drain AP, Barrett AS et al (2021) Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat Mater 20:548–559
Zhai Q, Fan J, Lin Q et al (2019) Tumor stromal type is associated with stromal PD-L1 expression and predicts outcomes in breast cancer. PLoS One 14:e0223325
Park JH, McMillan DC, Powell AG et al (2015) Evaluation of a tumor microenvironment-based prognostic score in primary operable colorectal cancer. Clin Cancer Res 21:882–888
Hwang HW, Kim JY, Lee SE et al (2020) Prognostic effects of histology-based tumour microenvironment scores in resected distal bile duct cancer. Histopathology 77:402–412
Park JH, van Wyk H, McMillan DC et al (2020) Preoperative, biopsy-based assessment of the tumour microenvironment in patients with primary operable colorectal cancer. J Pathol Clin Res 6:30–39
Acknowledgements
The authors would like to thank Bingning Wang, Hua Zeng, and Wenchao Liu for their assistance in the immunohistochemical staining involved in the current study.
Funding
The authors received no funding for this work.
Author information
Authors and Affiliations
Contributions
J.Y. and L.X. provide guidance and revise manuscripts. N.C. contributed to the methodology, data analysis, and writing; B.W. provided acquisition, analysis, and interpretation of data. J.X. contributed to the clinicopathological data acquisition. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, and individual consent was waived for this retrospective analysis.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

S Fig. 1
(PNG 173 kb)

S Fig. 2
(PNG 10626 kb)

S Fig. 3
(PNG 3616 kb)

S Fig. 4
(PNG 512 kb)

S Fig. 5
(PNG 403 kb)

S Fig. 6
(PNG 115 kb)

S Fig. 7
(PNG 1395 kb)

S Fig. 8
(PNG 152 kb)

S Fig. 9
(PNG 575 kb)

S Fig. 10
(PNG 705 kb)

S Fig. 11
(PNG 566 kb)

S Fig. 12
(PNG 1076 kb)

S Fig. 13
(PNG 770 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, N., Wang, B., Xu, J. et al. Tumor stroma ratio, tumor stroma maturity, tumor-infiltrating immune cells in relation to prognosis, and neoadjuvant therapy response in esophagogastric junction adenocarcinoma. Virchows Arch (2024). https://doi.org/10.1007/s00428-024-03755-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00428-024-03755-2