Abstract
According to the fifth edition of the World Health Organization (WHO) classification of tumors of the central nervous system (CNS), diffuse midline glioma H3 K27-altered is a grade 4 infiltrative glioma that arises from midline anatomical structures and is characterized by the loss of H3 K27me3 and co-occurring H3 K27M mutation or EZHIP overexpression. However, the H3 K27M mutation has also been observed in circumscribed gliomas and glioneuronal tumors arising in midline anatomical structures, which may result in diagnostic pitfalls.
Rosette-forming glioneuronal tumor (RGNT) is a CNS WHO grade 1 neoplasm that histologically features neurocytic and glial components and originates in midline anatomical structures.
This study aimed to assess whether RGNTs, similar to other midline tumors, may exhibit immunohistochemical loss of H3 K27me3 and harbor the H3 K27M mutation.
All seven analyzed RGNTs displayed immunohistochemical loss of H3 K27me3 in all tumor cells or H3 K27me3 mosaic immunostaining. In one case, H3 K27me3 loss was associated with the H3 K27M mutation, whereas the other six cases did not exhibit any H3 mutations or EZHIP overexpression. During a follow-up period of 23 months, the H3 K27M-mutant case remained unchanged in size despite partial resection, indicating that the H3 mutation may not confer higher biological aggressiveness to RGNT.
The immunohistochemical loss of H3 K27me3 co-occurring with the H3 K27M mutation may result in the potential misdiagnosis of RGNT, especially in cases of small biopsy specimens consisting of only the glial component.
Similar content being viewed by others
Introduction
In the fifth edition of the World Health Organization (WHO) classification of central nervous system (CNS) tumors, diffuse midline glioma H3 K27-altered is a pediatric-type diffuse high-grade glioma that arises in midline anatomical structures and displays the immunohistochemical loss of H3 trimethylated in lysine 27 (H3 K27me3), in association with H3 K27M mutation, or EZHIP overexpression [1]. These tumors preferentially occur in the brainstem, thalamus, or spinal cord and exceptionally in the pineal gland, hypothalamus, and cerebellum [2,3,4,5]. Regardless of the presence of histopathological features of malignancy, they are classified as CNS WHO grade 4 [1] because of their poor outcome [6]. However, H3 K27M mutation has also been reported in other tumors originated in midline anatomical structures and characterized by less aggressive clinical behavior, including pylocytic astrocytomas [7, 8], gangliogliomas [9], glioneuronal tumors not otherwise specified [10], and infratentorial IDH-mutant astrocytomas [11]. The presence of this genetic alteration in tumors other than H3 K27-altered diffuse midline gliomas may result in misdiagnosis. As it was already emphasized by the c-IMPACT-NOW (Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy—Not Official WHO) [12], the diagnosis of diffuse midline glioma H3 K27M-mutant should be reserved for gliomas that are infiltrating and originated in midline anatomical structures, and not be extended to other H3 K27M-mutant tumors [1]. Nevertheless, the immunohistochemical loss of H3 K27me3 or the detection of H3 K27M mutation may pose diagnostic challenges in small biopsy specimens, where the distinction between circumscribed and diffuse gliomas, or between glial and glioneuronal tumors, may be difficult.
Rosette-forming glioneuronal tumor (RGNT) is a CNS WHO grade 1 biphasic neoplasia that histologically features a component of neurocytic cells arranged in rosettes or perivascular pseudorosettes and a glial component with piloid or oligodendroglia-like cells resembling pylocitic astrocytoma [13]. This rare tumor primarily affects children, adolescents, and young adults, and can involve the fourth ventricle or aqueduct, the brainstem, cerebellar vermis, quadrigeminal plate, pineal gland, or thalamus [13]. RGNT displays a unique DNA methylation profile and distinct genetic features consisting of mutations in the tyrosine kinase domain (either at p.N546 or p.K656) of FGFR1, co-occurring mutations of either PIK3CA or PIK3R1, and in a subset of cases inactivation of NF1 [14, 15].
In this study, we investigated whether RGNTs, like other midline tumors, may show immunohistochemical loss of H3 K27me3 and harbor the H3 K27M mutation.
Materials and methods
Cases
This study included seven RGNTs obtained from six female and one male patient (aged 18–43 years, with a median age of 29 years). All tumors were localized in midline anatomical structures, including the mesencephalon (n = 3), pineal gland (n = 2), hypothalamus (n = 1), and Sylvian aqueduct (n = 1). On imaging, all seven tumors appeared relatively circumscribed; three were described as cystic-solid, whereas four were reported as solid masses (Table 1).
Gross total resection was achieved in only one case, whereas six underwent biopsy or partial resection. No patients had received adjuvant therapy.
Recurrence-free survival (RFS) data were retrieved from the clinical records.
Immunohistochemistry
Immunohistochemistry was performed in all cases using antibodies against H3 K27me3 (clone C36B11, Cell Signaling Technology, Danvers, MA, USA; dilution 1:200), Histone H3.3 K27M-mutant (polyclonal, Merck KGaA, Darmstadt, Germany; dilution 1:500), and EZHIP/CXorf67 (polyclonal, Merck KGaA, Darmstadt, dilution 1:75), and an automated immunostainer (Leica Biosystems, Newcastle, UK).
H3 K27me3 immunohistochemical expression was considered: (i) lost when immunostaining was absent in >95% neoplastic cells and present in the internal positive controls (endothelium, non-neoplastic cells) [16]; (ii) retained, when nuclear staining was present in >95% of tumor cells; and (iii) mosaic, when the percentage of immunostained neoplastic cells was between 5 and 95%. Histone H3.3 K27M-mutant and EZHIP immuno-expression was classified as present or absent in tumor cells.
Next-generation sequencing
Cases showing immunohistochemical loss of H3 p.K28me3 and/or Histone H3.3 K27M-mutant immuno-expression were further analyzed using a next-generation sequencing (NGS) panel targeting 523 cancer-relevant genes (TruSight Oncology 500, Illumina, San Diego, CA, USA).
Genomic DNA was extracted from FFPE tissue sections using Maxwell CSC instrument (Promega, Madison, USA) with Maxwell RSC DNA FFPE kit (Promega, Madison, USA) according to the manufacturer’s protocol; DNA concentrations were measured on a Qubit 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, USA) using the Qubit dsDNA High Sensitivity. DNA libraries were prepared using TSO500 Library Preparation Kit (Illumina, San Diego, CA, USA) and sequenced to a mean coverage depth of >500× for up to 500 cancer-related genes. NGS data were analyzed with Illumina TruSight Oncology 500 Local App v2.1 and variant report files were uploaded into the Pierian Clinical Genomics Workspace cloud (Pierian DX software CGW_V6.21.1).
Results
RGNTs have lost or mosaic H3 K27me3 immunostaining
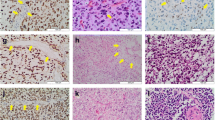
All RGNTs exhibited loss of H3 K27me3 or mosaic immunostaining. Three cases displayed immunohistochemical loss of H3K27me3 in the entirety of the tumor cells. One of these RGNTs showed positive immunostaining for Histone H3.3 K27M-mutant (case 1) (Fig. 1), whereas the other two cases were negative for both H3.3 K27M-mutant and EZHIP (Fig. 2).
RGNT displaying the immunohistochemical loss of H3K27me3 in the entirety of neoplastic cells, coupled with Histone H3 K27M-mutant positivity (case 1). H3 K27me3 immunohistochemical expression was lost in both the rosette-forming (left) and glial (right) components (endothelial cells retained the immunostaining and served as internal positive control). These latter exhibited Histone H3.3 K27M-mutant immunostaining and were negative for EZHIP. Next-generation sequencing confirmed the presence of H3.3 K27M mutation
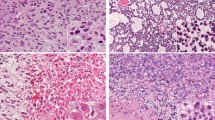
RGNT displaying the immunohistochemical loss of H3K27me3 in the entirety of neoplastic cells, in the absence of any staining for either Histone H3 K27M-mutant or EZHIP (case 2). H3 K27me3 immunohistochemical expression was lost in both the rosette-forming (left) and glial (right) components (endothelial cells retained the immunostaining and served as internal positive control). Both tumor components were negative for either Histone H3.3 K27M-mutant immunostaining or EZHIP. Next-generation sequencing did not reveal H3F3A, HIST1H3B, and HIST1H3C mutations
Four RGNTs had mosaic H3 K27me3 expression, with the percentage of neoplastic stained cells ranging between 40 and 60% (Table 1). In the tumor areas with H3 K27me3 loss, endothelial cells displayed strong staining for this protein (Fig. 3). Moreover, in female patients, negative tumor cells exhibited H3 K27me3 dot-like immunostaining in the inactivated X chromosome (Fig. 4). None of RGNTs with H3 K27me3 mosaic expression were positive for H3.3 K27M-mutant or EZHIP.
H3 K27M mutation may occur in RGNTs
All three RGNTs that showed loss of H3 K27me3 presented with mutations in FGFR1 at p.N546 or p.K656, and concurrent mutations in PIK3CA (Table 1). Of these cases, the one with positive staining for H3.3 K27M-mutant (case 1) harbored the K27M mutation in H3F3A, whereas the other two cases did not have any mutations in H3F3A, HIST1H3B, and HIST1H3C.
The follow-up period for the entire cohort ranged from 5 to 134 months, with a median of 27 months. None of the patients experienced recurrence or re-growth of their tumor. The RGNT with the K27M mutation in H3F3A remained stable in size over a 23-month follow-up period (Table 1).
Discussion
Since the description of diffuse midline glioma H3 K27M-mutant as a distinct tumor type characterized by a dismal prognosis [17], there have been reports of the H3 K27M mutation occurring in other tumors. In 2018, a systematic review and meta-analysis of the literature published between 2012 and 2017, conducted by Pratt et al., identified 26 H3 K27M-mutant circumscribed gliomas originating in midline anatomical structures [18]. The histological diagnoses of these cases included pilocytic astrocytoma, ganglioglioma, anaplastic ganglioglioma, glioneuronal tumor, anaplastic glioneuronal tumor, ganglion cell tumor, anaplastic ependymoma, and circumscribed glioma not otherwise specified [18]. The analysis of survival data, available for 21 cases, showed that patients with circumscribed gliomas H3 K27M-mutant had longer overall survival compared to patients with both H3 K27-altered and H3-wild-type diffuse gliomas, but shorter overall survival than patients with H3-wild-type circumscribed gliomas [18]. These results suggest that the H3 K27M mutation might be a characteristic feature of tumors arising in midline anatomical structures and likely confers higher biological aggressiveness even to circumscribed gliomas compared to cases lacking this genetic alteration [18]. Similarly, the H3 K27M mutation was associated with worse prognosis in infratentorial IDH-mutant astrocytomas [11]. However, due to reports of pilocytic astrocytomas, glioneuronal tumors, or subependymomas with a favorable prognosis despite the H3 K27M mutation [8,9,10, 19], the prognostic significance of the H3 K27M mutation in tumors other than diffuse gliomas remains unclear.
To the best of our knowledge, this is the first study to report a H3 K27M mutation in RGNT. In this particular case, the tumor cells showed positive immunostaining for the Histone H3.3 K27M-mutant and a concordant immunohistochemical loss of H3 K27me3. The H3 K27M mutation was confirmed by NGS, which also identified three mutations in the tyrosine kinase domain of FGFR1 (amino acids 478–767) and a mutation in PIK3CA. Over the course of 23 months, the tumor has demonstrated no evidence of regrowth, and has remained unchanged in size. This suggests that RGNTs with the H3 K27M mutation do not exhibit biological behavior consistent with that of high-grade tumors.
Another novel finding of this study was that all seven analyzed RGNTs exhibited a loss of immunohistochemical H3 K27me3 in at least a proportion of cells, with three cases displaying the absence of H3 K27me3 in the entirety of the tumor cells. A previous study analyzed the pattern of H3 K27me3 immunostaining in two RGNTs and both retained the expression of this protein, although the percentage of positive cells was not detailed [20]. Notably, the RGNTs with mosaic expression displayed strong H3K27me3 immunostaining in the endothelial cells within the areas with loss of staining in the neoplastic cells, thus ruling out a technical artifact. A similar pattern of H3 K27me3 immunostaining was reported in 22 out of 62 (35%) atypical meningiomas [21]. Further substantiating that this staining pattern is not artifactual, in tumors from female patients, the negative tumor cells displayed H3 K27me3 dot-like immunostaining in the inactivated X chromosome, as previously described in diffuse gliomas [22].
The loss of trimethylation of lysine 27 in the H3 protein may result from the H3 K27M mutation, which hinders the biochemical inhibition of the Polycomb Repressor Complex 2 (PRC2) [6]. Alternatively, it may originate from EZHIP overexpression, which inhibits the functional enzymatic component EZH2 of PRC2 [23]. However, excluding the case harboring the K27M mutation in H3F3A, none of the other six RGNTs had mutations in histone genes or EZHIP overexpression, suggesting that H3 K27me3 loss may be attributed to other mechanisms that impede PRC2 function.
H3 K27me3 immunohistochemical loss has been observed in various CNS tumors, regardless of their location in midline anatomical structures. This loss of H3 K27me3 expression has been reported to predict a poor prognosis in ependymomas occurring in the posterior fossa [24] as well as a shorter time to recurrence and resistance to radiosurgery in meningiomas [25]. However, in diffuse IDH-mutant hemispheric gliomas, the immunohistochemical loss of H3 K27me3 is associated with a significantly better prognosis [16]. In this study, none of the RGNTs experienced regrowth or recurrence over a range of 5 to 134 months. Although some cases had limited follow-up time, these findings suggest that H3 K27me3 loss has no prognostic significance in RGNTs. Recently, Kim et al. have reported that H3 K27me3 immunoexpression was lost in all 25 central neurocytomas and three pituicytomas analyzed in absence of H3F3A mutations and EZHIP overexpression [26]. Therefore, the spectrum of tumors with loss of trimethylation at lysine 27 of H3 is broader than initially believed and encompasses diverse tumor types arising from midline structures through different pathogeneses.
In conclusion, this study is the first to demonstrate that the H3 K27M mutation and immunohistochemical loss of H3 K27me3 may characterize RGNTs. This should be acknowledged to avoid misdiagnosis as diffuse midline glioma H3 K27-altered, especially in the case of small biopsy specimens of RGNT showing the sole glial component of the tumor, and where the absence of infiltrating features may not be easily discernible. In these cases, radiological or intra-operative impression of a well-circumscribed tumor may be helpful in the differential diagnosis. Indeed, all seven cases in this cohort appeared to be relatively circumscribed on imaging, as previously reported in other series [27], and five were described as well-demarcated in the surgery reports as well. In addition, DNA methylation profiling or genetic analyses involving FGFR1, PIK3CA, and PIK3R1 may aid in resolving the differential diagnosis of H3 K27-altered diffuse gliomas. Indeed, co-occurring mutations in FGFR1 and PIK3CA or PIK3R1 were evidenced in the majority of RGNTs [14, 15, 28]. On the other hand, the co-occurrence of these mutations was exceptionally observed in H3 K27-altered diffuse gliomas [29,30,31].
Data availability
Data will be available upon request to corresponding author.
References
Varlet P, Ellison DW, Solomon DA, Suvà ML, Baker SJ, Jabado N, Jones DTW, Jones C, Orr BA, Warren KE, Leske H (2021) Diffuse midline glioma, H3 K27-altered. In: Hawkins C, Pfister SM (eds) Central Nervous System Tumours. International Agencgy for Research on Cancer, Lyon
Gilbert AR, Zaky W, Gokden M, Fuller CE, Ocal E, Leeds NE, Fuller GN (2018) Extending the neuroanatomic territory of diffuse midline glioma, K27M mutant: pineal region origin. Pediatr Neurosurg 53:59–63. https://doi.org/10.1159/000481513
Meyronet D, Esteban-Mader M, Bonnet C, Joly MO, Uro-Coste E, Amiel-Benouaich A, Forest F, Rousselot-Denis C, Burel-Vandenbos F, Bourg V, Guyotat J, Fenouil T, Jouvet A, Honnorat J, Ducray F (2017) Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol 19:1127–1134. https://doi.org/10.1093/neuonc/now274
Roux A, Pallud J, Saffroy R, Edjlali-Goujon M, Debily MA, Boddaert N, Sanson M, Puget S, Knafo S, Adam C, Faillot T, Cazals-Hatem D, Mandonnet E, Polivka M, Dorfmuller G, Dauta A, Desplanques M, Gareton A, Pages M et al (2020) High-grade gliomas in adolescents and young adults highlight histomolecular differences from their adult and pediatric counterparts. Neuro Oncol 22:1190–1202. https://doi.org/10.1093/neuonc/noaa024
Solomon DA, Wood MD, Tihan T, Bollen AW, Gupta N, Phillips JJ, Perry A (2016) Diffuse midline gliomas with histone H3-K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol 26:569–580. https://doi.org/10.1111/bpa.12336
Castel D, Kergrohen T, Tauziede-Espariat A, Mackay A, Ghermaoui S, Lechapt E, Pfister SM, Kramm CM, Boddaert N, Blauwblomme T, Puget S, Beccaria K, Jones C, Jones DTW, Varlet P, Grill J, Debily MA (2020) Histone H3 wild-type DIPG/DMG overexpressing EZHIP extend the spectrum diffuse midline gliomas with PRC2 inhibition beyond H3-K27M mutation. Acta Neuropathol 139:1109–1113. https://doi.org/10.1007/s00401-020-02142-w
Morita S, Nitta M, Muragaki Y, Komori T, Masui K, Maruyama T, Ichimura K, Nakano Y, Sawada T, Koriyama S, Tsuzuki S, Yasuda T, Hashimoto K, Niwa A, Kawamata T (2018) Brainstem pilocytic astrocytoma with H3 K27M mutation: case report. J Neurosurg 129:593–597. https://doi.org/10.3171/2017.4.JNS162443
Hochart A, Escande F, Rocourt N, Grill J, Koubi-Pick V, Beaujot J, Meignan S, Vinchon M, Maurage CA, Leblond P (2015) Long survival in a child with a mutated K27M-H3.3 pilocytic astrocytoma. Ann Clin Transl Neurol 2:439–443. https://doi.org/10.1002/acn3.184
Pages M, Beccaria K, Boddaert N, Saffroy R, Besnard A, Castel D, Fina F, Barets D, Barret E, Lacroix L, Bielle F, Andreiuolo F, Tauziede-Espariat A, Figarella-Branger D, Puget S, Grill J, Chretien F, Varlet P (2018) Co-occurrence of histone H3 K27M and BRAF V600E mutations in paediatric midline grade I ganglioglioma. Brain Pathol 28:103–111. https://doi.org/10.1111/bpa.12473
Orillac C, Thomas C, Dastagirzada Y, Hidalgo ET, Golfinos JG, Zagzag D, Wisoff JH, Karajannis MA, Snuderl M (2016) Pilocytic astrocytoma and glioneuronal tumor with histone H3 K27M mutation. Acta Neuropathol Commun 4:84. https://doi.org/10.1186/s40478-016-0361-0
Banan R, Stichel D, Bleck A, Hong B, Lehmann U, Suwala A, Reinhardt A, Schrimpf D, Buslei R, Stadelmann C, Ehlert K, Prinz M, Acker T, Schittenhelm J, Kaul D, Schweizer L, Capper D, Harter PN, Etminan N et al (2020) Infratentorial IDH-mutant astrocytoma is a distinct subtype. Acta Neuropathol 140:569–581. https://doi.org/10.1007/s00401-020-02194-y
Louis DN, Giannini C, Capper D, Paulus W, Figarella-Branger D, Lopes MB, Batchelor TT, Cairncross JG, van den Bent M, Wick W, Wesseling P (2018) cIMPACT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol 135:639–642. https://doi.org/10.1007/s00401-018-1826-y
Hainfellner JA, Jacques TS, Jones DTW, Rosenblum MK, Sievers P (2021) Rosette-forming glioneuronal tumor. In: WCoTE B (ed) Central Nervous system Tumours. IARC press, Lyon, pp 133–135
Sievers P, Appay R, Schrimpf D, Stichel D, Reuss DE, Wefers AK, Reinhardt A, Coras R, Ruf VC, Schmid S, de Stricker K, Boldt HB, Kristensen BW, Petersen JK, Ulhoi BP, Gardberg M, Aronica E, Hasselblatt M, Bruck W et al (2019) Rosette-forming glioneuronal tumors share a distinct DNA methylation profile and mutations in FGFR1, with recurrent co-mutation of PIK3CA and NF1. Acta Neuropathol 138:497–504. https://doi.org/10.1007/s00401-019-02038-4
Lucas CG, Gupta R, Doo P, Lee JC, Cadwell CR, Ramani B, Hofmann JW, Sloan EA, Kleinschmidt-DeMasters BK, Lee HS, Wood MD, Grafe M, Born D, Vogel H, Salamat S, Puccetti D, Scharnhorst D, Samuel D, Cooney T et al (2020) Comprehensive analysis of diverse low-grade neuroepithelial tumors with FGFR1 alterations reveals a distinct molecular signature of rosette-forming glioneuronal tumor. Acta Neuropathol Commun 8:151. https://doi.org/10.1186/s40478-020-01027-z
Ammendola S, Caldonazzi N, Simbolo M, Piredda ML, Brunelli M, Poliani PL, Pinna G, Sala F, Ghimenton C, Scarpa A, Barresi V (2021) H3K27me3 immunostaining is diagnostic and prognostic in diffuse gliomas with oligodendroglial or mixed oligoastrocytic morphology. Virchows Arch 479:987–996. https://doi.org/10.1007/s00428-021-03134-1
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Pratt D, Natarajan SK, Banda A, Giannini C, Vats P, Koschmann C, Mody R, Chinnaiyan A, Venneti S (2018) Circumscribed/non-diffuse histology confers a better prognosis in H3K27M-mutant gliomas. Acta Neuropathol 135:299–301. https://doi.org/10.1007/s00401-018-1805-3
Argenziano MG, Furnari JL, Miller ML, Sun Y, Banu MA, Neira JA, Snuderl M, Bruce JN, Welch M, McCormick P, Canoll P (2022) Thoracic low grade glial neoplasm with concurrent H3 K27M and PTPN11 mutations. Acta Neuropathol Commun 10:64. https://doi.org/10.1186/s40478-022-01340-9
Huang XD, Zhang HW, Feng YN, Lei Y, Lin F (2022) Rosette-forming glioneuronal tumours: two case reports and a review of the literature. BJR Case Rep 8:20210125. https://doi.org/10.1259/bjrcr.20210125
Vaubel RA, Kumar R, Weiskittel TM, Jenkins S, Dasari S, Uhm JH, Lachance DH, Brown PD, Van Gompel JJ, Jenkins RB, Kipp BR, Sukov WR, Giannini C, Johnson DR, Raghunathan A (2023) Genomic markers of recurrence risk in atypical meningioma following gross total resection. Neurooncol Adv 5:vdad004. https://doi.org/10.1093/noajnl/vdad004
Tschui J, Hewer E (2018) H3K27me3 immunostaining for sex determination in the context of presumed tissue misidentification. Histopathology 72:358–359. https://doi.org/10.1111/his.13364
Beringer M, Pisano P, Di Carlo V, Blanco E, Chammas P, Vizan P, Gutierrez A, Aranda S, Payer B, Wierer M, Di Croce L (2016) EPOP functionally links elongin and polycomb in pluripotent stem cells. Mol Cell 64:645–658. https://doi.org/10.1016/j.molcel.2016.10.018
Panwalkar P, Clark J, Ramaswamy V, Hawes D, Yang F, Dunham C, Yip S, Hukin J, Sun Y, Schipper MJ, Chavez L, Margol A, Pekmezci M, Chung C, Banda A, Bayliss JM, Curry SJ, Santi M, Rodriguez FJ et al (2017) Immunohistochemical analysis of H3K27me3 demonstrates global reduction in group—a childhood posterior fossa ependymoma and is a powerful predictor of outcome. Acta Neuropathol 134:705–714. https://doi.org/10.1007/s00401-017-1752-4
Ammendola S, Rizzo PC, Longhi M, Zivelonghi E, Pedron S, Pinna G, Sala F, Nicolato A, Scarpa A, Barresi V (2022) The immunohistochemical loss of H3K27me3 in intracranial meningiomas predicts shorter progression-free survival after stereotactic radiosurgery. Cancers (Basel) 14. https://doi.org/10.3390/cancers14071718
Kim H, Lee K, Shim YM, Kim EE, Kim SK, Phi JH, Park CK, Choi SH, Park SH (2023) Epigenetic alteration of H3K27me3 as a possible oncogenic mechanism of central neurocytoma. Lab Invest 103:100159. https://doi.org/10.1016/j.labinv.2023.100159
Gao L, Han F, Jin Y, Xiong J, Lv Y, Yao Z, Zhang J (2018) Imaging features of rosette-forming glioneuronal tumours. Clin Radiol 73:275–282. https://doi.org/10.1016/j.crad.2017.10.011
Appay R, Bielle F, Sievers P, Barets D, Fina F, Boutonnat J, Adam C, Gauchotte G, Godfraind C, Lhermitte B, Maurage CA, Meyronet D, Mokhtari K, Rousseau A, Tauziede-Espariat A, Tortel MC, Uro-Coste E, Burel-Vandenbos F, Chotard G et al (2022) Rosette-forming glioneuronal tumours are midline, FGFR1-mutated tumours. Neuropathol Appl Neurobiol 48:e12813. https://doi.org/10.1111/nan.12813
Dufour C, Perbet R, Leblond P, Vasseur R, Stechly L, Pierache A, Reyns N, Touzet G, Le Rhun E, Vinchon M, Maurage CA, Escande F, Renaud F (2020) Identification of prognostic markers in diffuse midline gliomas H3K27M-mutant. Brain Pathol 30:179–190. https://doi.org/10.1111/bpa.12768
Fontebasso AM, Papillon-Cavanagh S, Schwartzentruber J, Nikbakht H, Gerges N, Fiset PO, Bechet D, Faury D, De Jay N, Ramkissoon LA, Corcoran A, Jones DT, Sturm D, Johann P, Tomita T, Goldman S, Nagib M, Bendel A, Goumnerova L et al (2014) Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat Genet 46:462–466. https://doi.org/10.1038/ng.2950
Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, Baugh J, Chaney B, Hoffmann M, Lane A, Fuller C, Miles L, Hawkins C, Bartels U, Bouffet E, Goldman S, Leary S, Foreman NK, Packer R, Warren KE, Broniscer A, Kieran MW et al (2018) Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (DIPG): a collaborative report from the International and European Society for Pediatric Oncology DIPG Registries. J Clin Oncol 36:1963–1972. https://doi.org/10.1200/JCO.2017.75.9308
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement. Support was provided by the FUR 2023, University of Verona, Italy, to V. B.
Author information
Authors and Affiliations
Contributions
E.M.: original draft; revision of histological slides; analysis of immunohistochemical slides; data collection; interpretation of the results.
S.A.: revision of histological slides; data collection; interpretation of the results; reviewing and editing.
S.R.: interpretation of the results; original draft; reviewing and editing.
I.G.: molecular analysis; interpretation of the results; reviewing and editing.
G.B.: collection of data; review of immunohistochemical slides; interpretation of the results; reviewing and editing.
B.M.: collection of clinical data; reviewing and editing.
A.F.: collection of clinical data; reviewing and editing.
V.B.: conceptualization; revision of the histological slides; analysis of immunohistochemical slides; interpretation of the results; funding acquisition; reviewing and editing.
Corresponding author
Ethics declarations
Ethics approval
All data related to the cases in this study were deidentified.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marastoni, E., Ammendola, S., Rossi, S. et al. H3 K27M mutation in rosette-forming glioneuronal tumors: a potential diagnostic pitfall. Virchows Arch (2024). https://doi.org/10.1007/s00428-024-03739-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00428-024-03739-2