Abstract
Programmed death-ligand 1 (PD-L1) is overexpressed in cervical carcinoma, hindering tumor destruction. The aim of this study was to assess PD-L1 expression by immunohistochemistry in cervical squamous cell carcinoma (SCC) and squamous intraepithelial lesions (SILs) from human immunodeficiency virus–positive (HIV+) and human immunodeficiency virus-negative (HIV-) patients. A total of 166 SCC and SIL samples of HIV+ and HIV- patients were included and analyzed for PD-L1 expression through tumor proportion score (TPS), and results were stratified in five TPS groups using SP263 antibody and, combined positive score (CPS) using 22C3 antibody. In cohort 1 (SP263 clone), all HIV+ patients were negative for intraepithelial lesion or malignancy (NILM), and low-grade squamous intraepithelial lesions (LSILs) scored < 1; and 87.5% of high-grade squamous intraepithelial lesions (HSILs) adjacent to SCC, 19% of HSILs non-adjacent to SCC, and 69% of SCCs scored ≥ 1 (15.4% scored 5). In HIV- patients, all NILM, LSILs, HSILs adjacent to SCC, and two HSILs non-adjacent to SCC scored < 1. SCC: 88.2% scored ≥ 1 and 5.9% scored 5. In cohort 2 (SP263 and 22C3 clones), 16.7% of HIV+ patients with SCC were positive with both clones, CPS ≥ 1 (22C3) or score 5 (≥ 50%) (SP263), showing no significant differences in positivity between both clones. These results indicate that a relatively low percentage of SCCs (16.7%; both in HIV+ and in HIV- patients) express PD-L1 (TPS ≥ 50% and CPS > 1), which may be due to some samples being archival material, sample characteristics, or use of different methodologies, highlighting the need for standardization of PD-L1 assessment in SCC of the cervix. The fact that PD-L1 is overexpressed in SILs of HIV+ patients suggests potential additional applications for immunotherapy in this disease.
Similar content being viewed by others
Introduction
The role of immune checkpoint inhibitors has been explored in several malignancies, including uterine cervical cancer [1,2,3] due to their ability to interfere with the body’s defense mechanisms against cancer, but this role has been scarcely studied in the progression from squamous intraepithelial lesions (SILs) to squamous cell carcinoma (SCC).
Programmed cell death protein-1 (PD-1) and its ligand programmed death-ligand 1 (PD-L1) are coinhibitory regulators that suppress proliferation and cytokine production by CD8+ T lymphocytic cells, preventing tumor surveillance and destruction by immune cells [4].
Alterations in the PD-1/PD-L1 axis are implicated in the impairment of local immune response to HPV-associated cervical lesions. Immunohistochemistry (IHC) analysis in cervical intraepithelial neoplasia and cervical cancer revealed no PD-L1 expression in normal cervical epithelium (even when adjacent to cancer), but conversely showed PD-L1 expression in 95% of SILs and 80% of cervical SCCs (both in epithelial squamous and immune cells) [5]. Moreover, in patients with cervical cancer, an association was found between HPV-infected cells, increased number of CD8+ T-cells, and increased PD-L1 and PD-1 expression in the setting of progression from normal cervical epithelium to invasive cervical cancer [5,6,7,8,9].
CD8+ T lymphocytes are the primary inflammatory cells expressing PD-L1 and are strongly clustered around SILs and nests of invasive squamous cancer cells, suggesting that PD-L1 is upregulated in carcinoma and cervical cancer microenvironment [5].
Besides inhibiting T-cell proliferation and cytokine production, PD-L1 also promotes T-cell activation, although the explanation for this paradox remains elusive [10,11,12,13].
In chronic human immunodeficiency virus (HIV) infection, HIV-1 virions induce high levels of PD-L1 expression and resulting in an inhibitory effect on immune cells, like T-cells and neutrophils [14]. In addition, PD-L1 is expressed in CD4+Foxp3+ regulatory T-cells (T regs), which play an important role in promoting immunosuppression [15].
This may explain why the local immune response is not effective in preventing the development of cervical SCC in patients with HIV-positive (HIV+) infection and HPV-associated cervical epithelial lesions.
The rates of IHC detection of PD-L1 positivity in cervical specimens from patients not infected with HIV vary between 32% and 80% [5, 16, 17]. This high variation has been attributed to several factors, such as tissue sample characteristics (SILs vs. invasive cancer), cancer stage (early vs. metastatic), history of chemotherapy treatment and combination antiretroviral therapy (cART)–treated patients exhibiting a lower prevalence of PD-L1 immunopositivity [18]. In addition, the antibodies, quantification methods, and thresholds employed have also been acknowledged as important factors in PD-L1 tissue expression variation and it is unanimous among the scientific community the need for PD-L1 histopathological testing validation and standardization [19].
The primary aim of this study was to assess PD-L1 expression in cervical lesions from HIV+ patients treated in Portugal. SIL and SCC specimens were used to investigate PD-L1 expression in the context of tumor progression from the initial carcinogenesis steps to invasive carcinoma in HIV+ versus HIV- patients. The study’s secondary aim was to compare two different approaches to PD-L1 IHC assessment in invasive carcinoma using different antibodies and scoring systems.
Material and methods
This was an observational, cross-sectional study of HIV+ and HIV- patients with SILs or SCC followed at two health institutions in Portugal: Hospital Garcia de Orta, in Almada, and Instituto Português de Oncologia de Lisboa Francisco Gentil, in Lisbon.
Two patient cohorts were included. To address the primary study objective, a cohort of patients with SILs and SCC was included (cohort 1), and the SP263 antibody (Roche/Ventana) was used for IHC analysis. To address the secondary study objective, a second cohort of only SCC patients was included (cohort 2), and two antibodies were used for IHC analysis: SP263 (Roche/Ventana) and 22C3 (Dako). Samples from HIV+ and HIV- patients were included in both cohorts.
Cohort 1 comprised 140 cervical specimens of SILs and invasive carcinoma, of which 70 were from HIV+ (1 hysterectomy and 69 biopsies) and 70 from HIV- (61 biopsies and nine cone biopsies) patients. These specimens were assessed by IHC using the anti-PD-L1 antibody SP263. HIV+ samples comprised 13 invasive SCCs, eight high-grade squamous intraepithelial lesions (HSILs) adjacent to SCC, 21 HSILs non-adjacent to SCC, 20 low-grade squamous intraepithelial lesions (LSILs), and 20 negative for intraepithelial lesion or malignancy (NILM) samples. HIV- samples included 17 invasive SCCs, 9 HSILs adjacent to SCC, 20 HSILs non-adjacent to SCC, 20 LSILs, and 20 NILM. All slides were double-checked to confirm the diagnosis.
Cohort 2 comprised 35 SCC specimens, of which 18 were from HIV+ and 17 from HIV- patients. Half of invasive SCC cases (n = 9) were also included in the first cohort. IHC assessment was performed using the anti-PD-L1 antibody 22C3. HIV+ samples comprised 15 biopsies, one hysterectomy, and two cone resections, and HIV- samples comprised one biopsy and 16 cone resections. All slides were double-checked to confirm the diagnosis.
Immunohistochemistry methodology
IHC was performed on 4-μm-thick sections. All slides were stained on the BenchMark ULTRA IHC/ISH 136 Automatic staining platform (Ventana Medical Systems) using appropriate positive and negative controls.
PD-L1 qualitative immunohistochemical assay was conducted using the anti-human PD-L1 monoclonal antibody SP263 (Roche/Ventana) following the manufacturer’s instructions: 16-min incubation at 36 °C in combination with Ventana CC1 (64 min). The anti-human PD-L1 monoclonal antibody 22C3 (Dako) was used at a 1:40 dilution for 28 min with pre-treatment ULTRA CC1 for 48 min (catalogue number M3653, Agilent Dako). Antigen detection was performed with the OptiView DAB IHC Detection Kit (Ventana Medical Systems) using diaminobenzidine as chromogen for detecting expression. Tissue sections were counterstained with Mayer’s hematoxylin.
Immunostaining assessment
SP263 expression was evaluated by immunostaining in neoplastic epithelial cells according to VENTANA PD-L1 (SP263) Assay Staining Guide after choosing one histological section with high staining for each type of lesion/area. Membrane staining in epithelial cells was considered positive if a brown color of strong or moderate intensity was observed in basolateral or all cell membrane, and the percentage of stained tumor cells in total tumor cells (or tumor proportion score, TPS) in the chosen area was estimated [20]. Based on cut-offs established in previous studies, results were stratified in five TPS groups according to the percentage of positive tumor cells: 0 (0%), 1 (≥ 1% and < 5%), 2 (≥ 5% and < 10%), 3 (≥ 10% and < 20%), 4 (≥ 20% and < 50%), and 5 (≥ 50%) [21].
22C3 expression was evaluated by immunostaining using the combined positive score (CPS) as a measure of expression, as reported in the KEYNOTE-158 trial [22]. This scoring method was approved by the Food and Drug Administration (FDA) as a companion test for determining eligibility for treatment with pembrolizumab in advanced cervical cancer. CPS evaluates the number of PD-L1-staining cells (tumor cells, lymphocytes, macrophages) relative to all viable tumor cells using the formula of total PD-L1 positive cells / total tumor cells (positive + negative) × 100. The maximum CPS score is defined as CPS 100. Any perceptible and convincing partial or complete linear membrane staining of viable tumor cells recognized as distinct from cytoplasmic staining at ×20 magnification is considered positive and included in the scoring method. A minimum of 100 viable tumor cells in the stained slide is required to consider the specimen adequate for PD-L1 assessment. Several areas of the slide (at least five) with tumor and immune cells should be evaluated. Likewise, any membrane and/or cytoplasmic staining of mononuclear inflammatory cells within tumor nests and/or adjacent supporting stroma is considered positive and included in the CPS numerator. Neutrophils, eosinophils, plasma cells, and immune cells associated with the tumor, in situ carcinoma, normal structures, or ulcers are excluded from the CPS score.
Statistical analysis was performed using GraphPad Prism Software (version 9 for Windows, GraphPad Software LLC).
Results
Cohort 1
A total of 168 different specimen areas were assessed with the SP263 anti-PD-L1 antibody using the previously defined five TPS groups according to the percentage of positive tumor cells (Table 1).
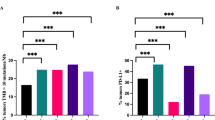
In HIV+ patients, 20 NILMs and 20 LSILs from 20 different cases had a TPS of 0. Among the 8 HSILs adjacent to invasive carcinoma from 8 different samples, one had a TPS of 0, four had a TPS of 1, and two had a TPS of 2 (7/8 [87.5%] areas scored ≥ 1), and among 21 HSILs non-adjacent to carcinoma from 21 different samples, 17 had a TPS of 0 and four had a TPS of 1 (4/21 [19%] areas scored ≥ 1) (Fig. 1). In SCC, four areas scored 0 and 9/13 areas (69%) scored ≥ 1, with the following distribution: two had a TPS of 1, one had a TPS of 2, one had a TPS of 3, three had a TPS of 4, and two (15.4%) had a TPS of 5 (Fig. 2). Cases with TPS of 4 and 5 corresponded to 38.5% of the total number of cases. In HIV- patients, all NILMs and LSILs from 40 different cases had a TPS of 0, as well as 9 HSILs adjacent to invasive carcinoma and 20 HSILs non-adjacent to carcinoma from 20 different cases.
In the 17 HIV- patients with invasive SCC, three had a TPS of 0 and the remaining 14 had a TPS ≥ 1. Among the latter, three had a TPS of 1, one had a TPS of 2, one had a TPS of 3, eight had a TPS of 4, and one (6%) had a TPS of 5. Cases with a TPS of 4 and 5 accounted for 41% of the total number of SCCs.
The number of SCC cases with a TPS of 5 was higher in the HIV+ (2/13 cases [15.4%]; Fig. 2) compared to the HIV- (1/17 cases [5.9%]) subgroup, although without statistical significance (p = 0.5645; Table 1).
Regarding HSILs adjacent to invasive SCC (Fig. 1), a significant difference was found in PD-L1 positivity when comparing HIV+ to HIV- patients, with HIV patients showing higher levels (p = 0.0004; Table 1). No significant differences were found in PD-L1 positivity (negative vs. positive) between the two groups for all the other types of lesions assessed.
In LSILs and NILMs, no area displayed PD-L1 positivity with the SP263 antibody (Table 1).
Cohort 2
The results of cohort 2 are depicted in Table 2. In HIV+ patients, SP263 antibody analysis of PD-L1 expression was positive in 66.7% (12/18) of SCCs (TPS ≥ 1), of which five cases had TPS = 0, one had TPS = 1, two had TPS = 2, six had TPS = 4, and two had TPS = 5 (11.1%). SCC cases with TPS of 4 or 5 accounted for 44.4% of the total number of cases.
Among the 17 HIV- patients with SCC, only two had a TPS of 0, with all the other cases (88.2%) scoring ≥ 1: three had TPS = 1, one had TPS = 2, one had TPS = 3, eight had TPS = 4, and one (5.9%) had TPS = 5. Cases with TPS of 4 or 5 accounted for 52.9% of the total invasive SCC cases.
Figure 3 shows the analysis with the 22C3 antibody. In HIV+ patients, PD-L1 expression was positive (CPS ≥ 1) in three out of 18 (16.6%) invasive SCC cases, with a CPS of 9, 6, and 1, respectively. In contrast, all invasive SCCs from HIV- patients (n = 17) were PD-L1 negative (CPS < 1). The three HIV+ cases that were positive for PD-L1 expression in the 22C3 antibody analysis (CPS ≥ 1) were also positive in the SP263 antibody analysis, showing high TPS (2 cases with TPS = 4, and 1 case with TPS = 5; Table 1).
In the SP263 antibody analysis, although more SCC cases were PD-L1-positive in the HIV- group (69.2% HIV+; 82.4% HIV-), no significant differences were found in PD-L1 positivity (TPS ≥ 1) between HIV+ and HIV- patients. Conversely, different results were found in the 22C3 antibody analysis, with a higher number of PD-L1-positive cases in the HIV+ SCC population. Positive CPS ≥ 1 (22C3) was found in 16.7% of SCC cases in HIV+ patients, compared to a null score in 0% of HIV- SCC cases (Table 1). However, when considering the highest PD-L1 threshold (TPS 5 [≥ 50%] for SP263 antibody), more cases were found in HIV+ compared to HIV- patients (15.4% vs. 5.9%).
Table 3 depicts the differences in PD-L1 expression in SCC of HIV+ and HIV- patients with 22C3 and SP263 antibodies. Using the 22C3 antibody, CPS ≥ 1 was found in three cases (16.7%) of SCC from HIV+ patients and none (0%) of SCC from HIV- patients, while CPS < 1 was found in 15 cases (83.3%) of SCC from HIV+ patients and 17 cases (100%) of SCC from HIV- patients. Using the SP263 antibody, TPS ≥ 1 was found in 12 cases (66.7%) of SCC from HIV+ patients and in 15 cases (88.2%) of SCC from HIV-patients, while TPS < 1 was found in 6 cases (33.3%) of SCC from HIV+ patients and in two cases (11.8%) of SCC from HIV- patients.
Considering only cases with CPS ≥ 1 or TPS 5 (≥ 50% positive tumor cells), 16.7% (3/18) of cases in the HIV+ group had CPS ≥ 1, and 16.7% (3/18) had TPS 5, showing a 100% agreement between both antibody analyses. In the HIV- group, 5.9% of cases had a TPS of 5, with no patients showing CPS ≥ 1, representing a slightly lower agreement of 94.1% between both analyses.
Discussion
Blockage of the immune response to cancer cells can be bypassed by drugs targeting this immune receptor [2, 17]. Strategies blocking the PD-L1 pathway have the potential to restore the antitumor activity of T-cells and thus be useful to treat malignant neoplasms [23].
The prognostic value of PD-L1 expression in patients diagnosed with invasive cervical carcinoma has been documented [24]. In cervical cancer, TGCA amplifications in CD274 (PD-L1) and PDCD1LG2 (PD-L2) immune targets were found to be associated with this type of cancer [25], and PD-L1 amplification and polysomy have been shown to represent valid prognostic biomarkers in the treatment with anti-PD-L1s [26].
To our knowledge, this is the first study evaluating PD-L1 expression in SCC and SILs from HIV+ patients in a Western population. Considering the TPS threshold of ≥ 50% for SP263 and CPS > 1 for 22C3, a similar percentage of PD-L1 positivity was found in HIV+ patients with both antibodies (16.7%), reaching a 100% positivity concordance between both. This percentage is however lower compared to other HIV+ series that have used the SP263 antibody: 31% positivity in HIV+ locally advanced cervical cancer (with a TPS ⩾ 25% threshold for positivity), and 60% positivity when considering the mean percentage of positive tumor cells [27].
A generally higher PD-L1 expression was found in HIV- patients with cervical carcinoma in this study compared to the study by Feng et al. (2018), which investigated 219 SCC patients using the CD274 antibody [16]. The authors reported a PD-L1 positivity of 32.4% (71/219) in cervical SCC, lower than the 82.4% observed in the present study with the SP263 antibody (TPS ≥ 1) in HIV- patients. However, when the 3+ staining threshold (which refers as positive to cases with strong membranous staining) was considered, Feng and colleagues found a considerably higher positivity rate (12.3%; 27/219) than the one found in the present study in HIV- patients: 6% with SP263 (TPS 5) and 0% with 2C23 [16].
The study by Heren et al. in 156 SCCs and 49 adenocarcinomas of the cervix using the E1L3N clone reported positive PD-L1 in 54% of SCC samples [28]. Positivity was assumed when ≥ 5% of tumor cells were positive for PD-L1 and results similar to ours were achieved in the HIV- population. Interestingly, they also showed that diffuse PD-L1 expression was a risk factor for poor disease-free and disease-specific survival outcomes compared to marginal PD-L1 expression on the interface between tumor and stroma, raising awareness of the importance of tumor microenvironment and suggesting that PD-L1 heterogeneity in tissue may at least partly explain the different PD-L1 positivity results reported in the literature.
The therapeutic inhibition of PD-L1 has been proposed in several studies, and its efficacy demonstrated in clinical trials of patients with recurrent or metastatic cervical cancer (KEYNOTE-028, KEYNOTE-158, CheckMate-358) [22, 29, 30]. The FDA approval of the PD-L1 inhibitor pembrolizumab is conditional on the assessment of PD-L1 expression in cancer tissues with a specific companion assay [2]. The use of companion assays for the assessment of PD-L1 expression is a relevant issue, due to the lack of standardization among assays.
Differences in PD-L1 positivity in different studies may be partly attributed to technical conditions (like the age of the paraffin block), to the number of cases included, or even to the percentage of patients under cART and/or different cART durations.
The variability of immunostaining techniques used by different laboratories in these procedures introduces a great level of uncertainty among pathologists [31], with PD-1/PD-L1 status considered an unreliable biomarker to predict patient response in clinical trials of SCC of the uterine cervix [32] and anal canal [33], and reinforces the need for assay standardization. Different strategies have been proposed for assay harmonization, including the use of calibrators [34, 35].
Thirty-five invasive SCC samples (18 HIV+ and 17 HIV-) were assessed in this study using the SP263 and 22C3 antibodies, in line with previous reports using SP263 in gynecologic cancers [18, 36] and 22C3 according to the FDA-approved companion assay for pembrolizumab [37], and considering the TPS threshold of ≥ 50% for SP263 and CPS > 1 for 22C3. A similar percentage of PD-L1 positivity was found in HIV+ patients with the two antibodies (16.7%), reaching a 100% positivity concordance between both.
PD-L1 positivity in cervical SCC was found to vary according to the antibody used, although the differences in the results obtained with SP263 and 22C3 antibodies were not significant (Table 3). Interestingly, Mills’ group validated the PD-L1 IHC assay using the SP263 antibody on Ventana BenchMark ULTRA against the 22C3 antibody and reported a 97% positivity concordance between both at a cut-off of 50% staining for SP263 and CPS ≥ 1 staining for 22C3 [27].
The value of PD-L1 expression has been explored in the HIV population with SCC in several tumor sites. Using the ab153991 antibody, Govindarajan et al. reported a 56% PD-L1 positivity in 41 SCC samples from the anal canal of HIV- patients, similar to what has been reported in cervical carcinoma [38]. Very interesting results were presented by Bushara and colleagues [39], who reviewed data of the high incidence of anal SCC in HIV+ patients (even under cART) and concluded that chronic inflammation due to HIV infection may contribute to increased PD-L1 expression and CD8+ T lymphocyte exhaustion, impairing the cytotoxic antitumor response as previously reported by our group [40]. In contrast to these findings, another study in HIV+ and HIV- patients diagnosed with SCC of the anal canal showed that HIV status was not correlated with PD-L1 expression or with the degree or composition of immune cell infiltration and found a significant increase in IL-18 expression in HIV-associated anal SCC [41].
Regarding premalignant lesions, higher PD-L1 positivity rates were found in this study in HIV+ patients with SCC versus SILs and with HSILs adjacent to invasive carcinoma versus HSILs non-adjacent to invasive carcinoma, in contrast with the negative PD-L1 scores found in HSILs (adjacent or non-adjacent to SCC) in the HIV- group. As far as the authors are aware, this result has not been reported in cervical lesions from HIV+ patients to date.
PD-L1 expression in cervical and anal SILs was investigated in three and two studies in the literature, respectively, with results showing a predominantly negative expression in normal epithelium and low to high expression in SILs [5, 9, 42,43,44]. However, most of these studies were conducted in the HIV- population. In the HIV+ population, only two studies have investigated PD-L1 expression in anal SILs [39, 41]. In the study by Bushara et al., HIV replication in immune cells was shown to increase the secretion of the tat protein that is integrated into epithelial cells of the HPV-infected anal canal, facilitating transcription of the HPV E6 and E7 oncoproteins and being responsible for the higher incidence of SILs in the anal canal of HIV+ patients, as observed in the cervix [39]. In the study by Yanik et al., PD-L1 expression was found to correlate with increased immune cell infiltration and CD8+ T-cell density in the immune microenvironment of the anal canal [41]. This finding is in line with previous observations from our group in cervical lesions of HIV+ patients showing that these patients present higher epithelial and stromal CD8+ T-cell scores in all cervical epithelial lesion groups (LSIL, HSIL, and SCC) compared to HIV- counterparts [40].
According to one study, PD-1-positive lymphocytes were more frequently observed in HSILs compared to LSILs in anal SCC of HIV+ patients, indicating that these alterations may be relevant for tumor progression and invasion [41]. Interestingly, a complementary observation was done in anal SILs of HIV- patients, suggesting that PD-1 and lymphocytic CD8+ T-cells are more commonly observed in high-grade lesions [42].
A clinical trial of the immune checkpoint inhibitor nivolumab in 37 patients with metastatic SCC of the anal canal showed the efficacy of this agent as monotherapy in these patients [43].
Findings of this study regarding PD-L1 expression in HSILs, especially in HIV+ patients, could be further explored, for instance regarding its use as a risk biomarker. As proposed by Brun et al., these lesions may be targeted by different types of therapeutic interventions interfering with checkpoint inhibitors, such as immunotherapy (e.g., peptide/protein-based vaccines, nucleic acid-based vaccines [DNA], and live vector-based vaccines [bacterial or viral]), just before lesion development [42].
Although the low number of cases included in this study is a limitation, the large number of positive PD-L1 cases identified with both antibodies in SCC of HIV+ patients sustain that HIV immunosuppression may have an impact in regulating checkpoint inhibitors, particularly since all patients were under cART. And, the fact that PD-L1 overexpression is observed in intraepithelial squamous cervical lesions in HIV+ patients suggests that it may be useful to explore broader immunotherapy applications in this disease, potentially relevant in earlier stages of carcinogenesis.
References
Han Y, Liu D, Li L (2020) PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res 10(3):727–742
Liu Y, Wu L, Tong R, Yang F, Yin L, Li M et al (2019) PD-1/PD-L1 inhibitors in cervical cancer. Front Pharmacol 10:65
Munari E, Mariotti FR, Quatrini L, Bertoglio P, Tumino N, Vacca P et al (2021) PD-1/PD-L1 in cancer: pathophysiological, diagnostic and therapeutic aspects. Int J Mol Sci 22(10):5123
Lim TS, Chew V, Sieow JL, Goh S, Yeong JP-S, Soon AL et al (2016) PD-1 expression on dendritic cells suppresses CD8 + T cell function and antitumor immunity. Oncoimmunology 5(3):e1085146
Mezache L, Paniccia B, Nyinawabera A, Nuovo GJ (2015) Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod Pathol 28(12):1594–1602
Heeren AM, Koster BD, Samuels S, Ferns DM, Chondronasiou D, Kenter GG et al (2015) High and interrelated rates of PD-L1+CD14+ antigen-presenting cells and regulatory T cells mark the microenvironment of metastatic lymph nodes from patients with cervical cancer. Cancer Immunol Res 3(1):48–58
Meng Y, Liang H, Hu J, Liu S, Hao X, Wong MSK et al (2018) PD-L1 expression correlates with tumor infiltrating lymphocytes and response to neoadjuvant chemotherapy in cervical cancer. J Cancer 9(16):2938–2945
Reddy OL, Shintaku PI, Moatamed NA (2017) Programmed death-ligand 1 (PD-L1) is expressed in a significant number of the uterine cervical carcinomas. Diagn Pathol 12(1):45
Yang W, Song Y, Lu Y-L, Sun J-Z, Wang H-W (2013) Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology 139(4):513–522
Dong H, Zhu G, Tamada K, Chen L (1999) B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 5(12):1365–1369
Tamura H, Dong H, Zhu G, Sica GL, Flies DB, Tamada K et al (2001) B7-H1 costimulation preferentially enhances CD28-independent T-helper cell function. Blood 97(6):1809–1816
Yamazaki T, Akiba H, Koyanagi A, Azuma M, Yagita H, Okumura K (2005) Blockade of B7-H1 on macrophages suppresses CD4+ T cell proliferation by augmenting IFN-γ-induced nitric oxide production. J Immunol 175(3):1586–1592
Yu J, Wang X, Teng F, Kong L (2016) PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther 9:5023–5039
Bowers NL, Helton ES, Huijbregts RPH, Goepfert PA, Heath SL, Hel Z (2014) Immune suppression by neutrophils in HIV-1 infection: role of PD-L1/PD-1 pathway. PLoS Pathog 10(3):e1003993
Francisco LM, Sage PT, Sharpe AH (2010) The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 236(1):219–242
Feng M, Xu L, He Y, Sun L, Zhang Y, Wang W (2018) Clinical significance of PD-L1 (CD274) enhanced expression in cervical squamous cell carcinoma. Int J Clin Exp Pathol 11(11):5370–5378
Saglam O, Conejo-Garcia J (2018) PD-1/PD-L1 immune checkpoint inhibitors in advanced cervical cancer. Integr Cancer Sci Ther 5(2):10.15761/ICST.1000272. https://doi.org/10.15761/ICST.1000272
Loharamtaweethong K, Vinyuvat S, Thammasiri J, Chitpakdee S, Supakatitham C, Puripat N (2019) Impact of antiretroviral drugs on PD-L1 expression and copy number gains with clinical outcomes in HIV-positive and -negative locally advanced cervical cancers. Oncol Lett 18(6):5747–5758
Torlakovic E, Lim HJ, Adam J, Barnes P, Bigras G, Chan AWH et al (2020) “Interchangeability” of PD-L1 immunohistochemistry assays: a meta-analysis of diagnostic accuracy. Mod Pathol 33(1):4–17
Roche (2019) VENTANA PD-L1 (SP263) Assay staining of non-small cell lung cancer interpretation guide. https://www.rochebiomarkers.be/content/media/Files/PD-L1_SP263_interpretation_guide_NSCLC.pdf. Accessed 20 Jan 2023
García A, Recondo G, Greco M, de la Vega M, Perazzo F, Recondo G, Avagnina A et al (2020) Correlation between PD-L1 expression (clones 28-8 and SP263) and histopathology in lung adenocarcinoma. Heliyon 6(6):e04117
Chung HC, Ros W, Delord J-P, Perets R, Italiano A, Shapira-Frommer R et al (2019) Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 37(17):1470–1478
Zhang L, Zhang M, Xu J, Li S, Chen Y, Wang W et al (2020) The role of the programmed cell death protein-1/programmed death-ligand 1 pathway, regulatory T cells and T helper 17 cells in tumor immunity: a narrative review. Ann Transl Med 8(22):1526–1526
Yang-Chun F, Zhen-Zhen C, Yan-Chun H, Xiu-Min M (2017) Association between PD-L1 and HPV status and the prognostic value for HPV treatment in premalignant cervical lesion patients. Medicine 96(25):e7270
The Cancer Genome Atlas Research Network (2017) Integrated genomic and molecular characterization of cervical cancer. Nature 543(7645):378–384
Loharamtaweethong K, Puripat N, Praditphol N, Thammasiri J, Tangitgamol S (2020) PD-L1 protein expression and copy number gains in HIV-positive locally advanced cervical cancer. Ther Adv Med Oncol 12:175883592096300
Mills AM (2021) PD-L1 interpretation in cervical carcinomas: Proceedings of the ISGyP Companion Society Session at the 2020 USCAP Annual Meeting. Int J Gynecol Pathol 40(1):1–4
Heeren AM, Punt S, Bleeker MC, Gaarenstroom KN, van der Velden J, Kenter GG et al (2016) Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod Pathol 29(7):753–763
Frenel J-S, Le Tourneau C, O’Neil B, Ott PA, Piha-Paul SA, Gomez-Roca C et al (2017) Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1–positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol 35(36):4035–4041
Naumann RW, Hollebecque A, Meyer T, Devlin M-J, Oaknin A, Kerger J et al (2019) Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II CheckMate 358 trial. J Clin Oncol 37(31):2825–2834
Martinez-Morilla S, Moutafi M, Rimm DL (2022) Standardization of PD-L1 immunohistochemistry. Mod Pathol 35(3):294–295
Rotman J, den Otter LAS, Bleeker MCG, Samuels SS, Heeren AM, Roemer MGM et al (2020) PD-L1 and PD-L2 expression in cervical cancer: regulation and biomarker potential. Front Immunol 11:596825
Mathias-Machado MC, Peixoto RD, Moniz CMV, Jácome AA (2022) Biomarkers in anal cancer: current status in diagnosis, disease progression and therapeutic strategies. Biomedicines 10(8):2029
Torlakovic EE, Sompuram SR, Vani K, Wang L, Schaedle AK, DeRose PC et al (2021) Development and validation of measurement traceability for in situ immunoassays. Clin Chem 67(5):763–771
Sompuram SR, Torlakovic EE, ’t Hart NA, Vani K, Bogen SA (2022) Quantitative comparison of PD-L1 IHC assays against NIST standard reference material 1934. Mod Pathol 35(3):326–332
Czogalla B, Pham D, Trillsch F, Rottmann M, Gallwas J, Burges A et al (2020) PD-L1 expression and survival in p16-negative and -positive squamous cell carcinomas of the vulva. J Cancer Res Clin Oncol 146(3):569–577
Slater H (2020) FDA approves PD-L1 IHC 22C3 pharmDx as companion diagnostic for pembrolizumab. https://www.cancernetwork.com/view/fda-approves-pd-l1-ihc-22c3-pharmdx-as-companion-diagnostic-for-pembrolizumab. Accessed 20 Jan 2023
Govindarajan R, Gujja S, Siegel ER, Batra A, Saeed A, Lai K et al (2018) Programmed cell death-ligand 1 (PD-L1) expression in anal cancer. Am J Clin Oncol 41(7):638–642
Bushara O, Krogh K, Weinberg SE, Finkelman BS, Sun L, Liao J et al (2022) Human immunodeficiency virus infection promotes human papillomavirus-mediated anal squamous carcinogenesis: an immunologic and pathobiologic review. Pathobiology 89(1):1–12
Brito MJ, Sequeira P, Silva I, Quintas A, Martins C, Félix A (2021) CD4+ and CD8+ cell populations in HIV-positive women with cervical squamous intra-epithelial lesions and squamous cell carcinoma. Int J Infect Dis 103:370–377
Yanik EL, Kaunitz GJ, Cottrell TR, Succaria F, McMiller TL, Ascierto ML et al (2017) Association of hiv status with local immune response to anal squamous cell carcinoma. JAMA Oncol 3(7):974
Brun J, Rajaonarison J, Nocart N, Hoarau L, Brun S, Garrigue I (2018) Targeted immunotherapy of high-grade cervical intra-epithelial neoplasia: expectations from clinical trials. Mol Clin Oncol 8(2):227–235
Bucau M, Gault N, Sritharan N, Valette E, Charpentier C, Walker F et al (2020) PD-1/PD-L1 expression in anal squamous intraepithelial lesions. Oncotarget 11(39):3582–3589
Yang W, Lu Y-P, Yang Y-Z, Kang J-R, Jin Y-D, Wang H-W (2017) Expressions of programmed death (PD)-1 and PD-1 ligand (PD-L1) in cervical intraepithelial neoplasia and cervical squamous cell carcinomas are of prognostic value and associated with human papillomavirus status. J Obstet Gynaecol Res 43(10):1602–1612
Acknowledgements
The authors acknowledge the clinical and technical staff of the contributing institutions for their help and support. The authors further acknowledge Joana Cavaco-Silva, Ph.D. (jo.cvsilva@gmail.com) for the medical writing and editorial assistance in the preparation of this manuscript.
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
MJB and AF designed the study.
AQ, PS, AF, and MJB selected the patients and samples included in the study.
IS and FS performed the IHC staining.
MJ and AF evaluated HER2 IHC staining and analyzed and interpreted the data.
MJB, CM, and AF drafted and revised the manuscript.
All the authors read, reviewed, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Ethics Committee of Hospital Garcia de Orta (HGO-CGO 23/2014), Institute of Oncology Francisco Gentil − Lisboa (IPO-UIC/996), and Nova Medical School (NMS-03/2015/CEFCM).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 18 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brito, M.J., Sequeira, P., Quintas, A. et al. Programmed death-ligand 1 (PD-L1) expression in cervical intraepithelial neoplasia and cervical squamous cell carcinoma of HIV-infected and non-infected patients. Virchows Arch 484, 507–516 (2024). https://doi.org/10.1007/s00428-023-03580-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-023-03580-z