Abstract
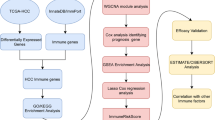
In recent years, breakthroughs in the field of tumor immunotherapy with immune checkpoint inhibitors (ICIs) have made a therapeutic revolution, which has been shown to improve the prognosis of patients with hepatocellular carcinoma (HCC). Immune infiltrates represent a major component of tumor microenvironment (TME), and play an essential role in both tumor progression and therapeutic response. The major unmet challenge in tumor immunotherapy is exploring the intrinsic and extrinsic mechanisms of TME promoting the management of HCC. Lysyl oxidase like 3 (LOXL3) participates in the remodeling of extracellular matrix (ECM) and the cross-linking of collagen and elastic fibers. It has been reported that LOXL3 is associated with the development and tumorigenesis of multiple types of cancer. RNA sequencing data and corresponding clinical information were extracted from The Cancer Genome Atlas (TCGA) databases, then subjected to gene expression, tumor microenvironment, survival, enrichment analyses utilizing R packages. In this study, we first found that LOXL3 gene was upregulated in tumor tissues compared with the normal tissues. Furthermore, LOXL3 expression is positively correlated with the infiltration of multiple immune cells and the expression of immune checkpoint genes in HCC. Meanwhile, high LOXL3 expression predicted poor outcomes of the patients with HCC. Functional enrichment analysis suggested that LOXL3 was mainly linked to extracellular structure and matrix organization, cell−cell adhesion, and T cell activation. This is the first comprehensive study to indicate that LOXL3 is correlated with immune infiltrates and may serve as a novel biomarker predicting prognosis and immunotherapy in HCC.








Similar content being viewed by others
Data availability
All data is available under reasonable request.
Abbreviations
- CAMs:
-
cell adhesion molecules
- CCLE:
-
cancer cell line encyclopedia
- CI:
-
confidence interval
- DEGs:
-
differentially expressed genes
- DSS:
-
disease-specific survival
- ECM:
-
extracellular matrix
- FC:
-
fold change
- FDR:
-
false discovery rate
- GEPIA:
-
gene expression profiling interactive analysis
- GO:
-
Gene Ontology
- GTEx:
-
genotype-tissue expression
- HBV:
-
hepatitis B virus
- HCC:
-
hepatocellular carcinoma
- HCV:
-
hepatitis C virus
- HR:
-
hazard ratio
- ICB:
-
immune checkpoint blockade
- ICIs:
-
immune checkpoint inhibitors
- IHC:
-
immunohistochemistry
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- LOX:
-
lysyl oxidase
- LOXL3:
-
lysyl oxidase like 3
- MMRs:
-
mismatch repair system
- NAFLD:
-
nonalcoholic fatty liver disease
- NCCN:
-
national comprehensive cancer network
- OS:
-
overall survival
- PD-1:
-
programmed death receptor 1
- PD-L1:
-
programmed death receptor ligand 1
- PPI:
-
protein–protein interaction
- TACE:
-
transarterial chemoembolization
- TCGA:
-
the cancer genome atlas
- THPA:
-
the human protein atlas
- TIMER:
-
tumor immune estimation resource
- TME:
-
tumor microenvironment
- VEGF:
-
vascular endothelial growth factor
References
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS (2021) Hepatocellular carcinoma. Nat Rev Dis Primers 7(1):6
Villanueva A (2019) Hepatocellular Carcinoma. N Engl J Med 380(15):1450–1462
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver (2018) EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 69(1):182–236
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 331(6024):1565–1570
Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, Signori E, Honoki K, Georgakilas AG, Amin A, Helferich WG, Boosani CS, Guha G, Ciriolo MR, Chen S, Mohammed SI, Azmi AS, Keith WN, Bilsland A, Bhakta D, Halicka D, Fujii H, Aquilano K, Ashraf SS, Nowsheen S, Yang X, Choi BK, Kwon BS (2015) Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol 35(Suppl):S185–S198
Rabinovich GA, Gabrilovich D, Sotomayor EM (2007) Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 25:267–296
Sangro B, Sarobe P, Hervás-Stubbs S, Melero I (2021) Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol:1–19
Molnar J, Fong KS, He QP et al (2003) Structural and functional diversity of lysyl oxidase and the LOX-like proteins. Biochim Biophys Acta 1647(1-2):220–224
Kagan HM, Li W (2003) Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem 88(4):660–672
Ye M, Song Y, Pan S, Chu M, Wang ZW, Zhu X (2020) Evolving roles of lysyl oxidase family in tumorigenesis and cancer therapy. Pharmacol Ther 215:107633
Laurentino TS, Soares R, Marie S, Oba-Shinjo SM (2019) LOXL3 function beyond amino oxidase and role in pathologies, including cancer. Int J Mol Sci 20(14)
Ribeiro AL, Kaid C, Silva P, Cortez BA, Okamoto OK (2017) Inhibition of lysyl oxidases impairs migration and angiogenic properties of tumor-associated pericytes. Stem Cells Int 2017:4972078
Jeong YJ, Park SH, Mun SH, Kwak SG, Lee SJ, Oh HK (2018) Association between lysyl oxidase and fibrotic focus in relation with inflammation in breast cancer. Oncol Lett 15(2):2431–2440
Kasashima H, Yashiro M, Okuno T, Miki Y, Kitayama K, Masuda G, Kinoshita H, Morisaki T, Fukuoka T, Hasegawa T, Sakurai K, Toyokawa T, Kubo N, Tanaka H, Muguruma K, Hirakawa K, Ohira M (2018) Significance of the lysyl oxidase members lysyl oxidase like 1, 3, and 4 in gastric cancer. Digestion. 98(4):238–248
Ren J, Wang X, Wei G, Meng Y (2021) Exposure to desflurane anesthesia confers colorectal cancer cells metastatic capacity through deregulation of miR-34a/LOXL3. Eur J Cancer Prev 30(2):143–153
Ye M, Zhou J, Gao Y, Pan S, Zhu X (2020) The prognostic value of the lysyl oxidase family in ovarian cancer. J Clin Lab Anal 34(12):e23538
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z (2017) GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 45(W1):W98–W102
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B, Liu XS (2017) TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res 77(21):e108–e110
Becht E, Giraldo NA, Lacroix L, Buttard B, Elarouci N, Petitprez F, Selves J, Laurent-Puig P, Sautès-Fridman C, Fridman WH, de Reyniès A (2016) Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol 17(1):218
Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, Gopalakrishnan V, Xi Y, Zhao H, Amaria RN, Tawbi HA, Cogdill AP, Liu W, LeBleu VS, Kugeratski FG, Patel S, Davies MA, Hwu P, Lee JE, Gershenwald JE, Lucci A, Arora R, Woodman S, Keung EZ, Gaudreau PO, Reuben A, Spencer CN, Burton EM, Haydu LE, Lazar AJ, Zapassodi R, Hudgens CW, Ledesma DA, Ong SF, Bailey M, Warren S, Rao D, Krijgsman O, Rozeman EA, Peeper D, Blank CU, Schumacher TN, Butterfield LH, Zelazowska MA, McBride KM, Kalluri R, Allison J, Petitprez F, Fridman WH, Sautès-Fridman C, Hacohen N, Rezvani K, Sharma P, Tetzlaff MT, Wang L, Wargo JA (2020) B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 577(7791):549–555
Aran D, Hu Z, Butte AJ (2017) xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol 18(1):220
Ru B, Wong CN, Tong Y, Zhong JY, Zhong SSW, Wu WC, Chu KC, Wong CY, Lau CY, Chen I, Chan NW, Zhang J (2019) TISIDB: an integrated repository portal for tumor-immune system interactions. Bioinformatics. 35(20):4200–4202
Cerretelli G, Ager A, Arends MJ, Frayling IM (2020) Molecular pathology of Lynch syndrome. J Pathol 250(5):518–531
Ortiz-Barahona V, Joshi RS, Esteller M (2020) Use of DNA methylation profiling in translational oncology. Semin Cancer Biol S1044-579X(20):30271–30276
Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G (2017) The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol 14(12):717–734
Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, Saw RP, Thompson JF (2012) Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol 30(21):2678–2683
Ingold Heppner B, Untch M, Denkert C, Pfitzner BM, Lederer B, Schmitt W, Eidtmann H, Fasching PA, Tesch H, Solbach C, Rezai M, Zahm DM, Holms F, Glados M, Krabisch P, Heck E, Ober A, Lorenz P, Diebold K, Habeck JO, Loibl S (2016) Tumor-infiltrating lymphocytes: a predictive and prognostic biomarker in neoadjuvant-treated HER2-positive breast cancer. Clin Cancer Res 22(23):5747–5754
Faivre S, Rimassa L, Finn RS (2020) Molecular therapies for HCC: looking outside the box. J Hepatol 72(2):342–352
Llovet JM, Montal R, Sia D, Finn RS (2018) Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 15(10):599–616
Greten TF, Wang XW, Korangy F (2015) Current concepts of immune based treatments for patients with HCC: from basic science to novel treatment approaches. Gut. 64(5):842–848
Pinter M, Scheiner B, Peck-Radosavljevic M (2021) Immunotherapy for advanced hepatocellular carcinoma: a focus on special subgroups. Gut. 70(1):204–214
Benson AB, Angelica MI et al (2021) Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw 19(5):541–565
Feng GS, Hanley KL, Liang Y, Lin X (2021) Improving the efficacy of liver cancer immunotherapy: the power of combined preclinical and clinical studies. Hepatology 73(Suppl 1):104–114
Bagaev A, Kotlov N, Nomie K et al (2021) Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell 39(6):845–865
Madden MZ, Rathmell JC (2021) The complex integration of T-cell metabolism and immunotherapy. Cancer Discov 11(7):1636–1643
Sanmamed MF, Nie X, Desai SS, Villaroel-Espindola F, Badri T, Zhao D, Kim AW, Ji L, Zhang T, Quinlan E, Cheng X, Han X, Vesely MD, Nassar AF, Sun J, Zhang Y, Kim TK, Wang J, Melero I, Herbst RS, Schalper KA, Chen L (2021) A burned-out CD8+ T-cell subset expands in the tumor microenvironment and curbs cancer immunotherapy. Cancer Discov 11:1700–1715
Acknowledgements
We acknowledge the TCGA, GTEx, CCLE, THPA, TIMER, TISIDB, GEPIA, and STRING databases for free use. Additionally, Ning Wang would like to thank his parents and wife for their encouragement and support in the scientific research work.
Author information
Authors and Affiliations
Contributions
Ning Wang and Xue Zhou contributed to the study conception, design, and visualization. Fei Tang and XiaoWei Zhu performed data analysis and figure generation. Xue Wang contributed to collection and integration of data. Ning Wang wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article is not involved in any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
All authors consent to the publication of this research.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Fig. S1
Identification of LOXL3 expression is in pan-cancer. a The expression of LOXL3 in 31 normal tissues based on GTEx database. b The expression of LOXL3 in 21 tumor cells based on CCLE database (PNG 613 kb)
Fig. S2
Correlation analysis of LOXL3 expression with the mutation levels of five MMR genes involving in MLH1, MSH2, MSH6, PMS2, and EPCAM in pan-cancer. *P < 0.05, **P < 0.01, and ***P < 0.001 (PNG 201 kb)
Fig. S3
Correlation analysis of LOXL3 expression with four DNA methyltransferase genes (DNMT1, red; DNMT2, blue; DNMT3A, green; DNMT3B, purple) in pan-cancer (PNG 1159 kb)
ESM 1
(XLSX 19 kb)
Rights and permissions
About this article
Cite this article
Wang, N., Zhou, X., Tang, F. et al. Identification of LOXL3-associating immune infiltration landscape and prognostic value in hepatocellular carcinoma. Virchows Arch 479, 1153–1165 (2021). https://doi.org/10.1007/s00428-021-03193-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-021-03193-4