Abstract
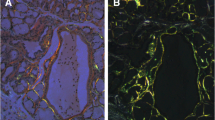
We analysed specificity and sensitivity of confocal laser microscopy (CLSM) on tissue sections for a diagnosis of amyloidosis, in an attempt to reduce technical errors and better standardise pathological diagnosis. We first set up a protocol for the use of CLSM on this type of specimen, using a group of 20 amyloid negative and 20 positive samples. Of all specimens, 2, 4 and 8-μm sections were cut. Sections were stained with Congo red (CR) and thioflavin-T (ThT) and observed by cross-polarised light microscopy (CR-PL), epifluorescence microscopy (CRF-epiFM and ThT-epiFM) and CLSM (CRF-CLSM and ThT-CLSM). To validate the method in a diagnostic setting, we examined tissue samples from 116 consecutive patients with clinical suspicion of amyloidosis, selected from the period 2005 to 2014 from the database of the Pathology Unit of the University of Padua. The results were compared with those of transmission electron microscopy (TEM), which we consider as reference. We found that with CRF-CLSM, the false negative rate was reduced from 17 to 5%, while the sensitivity of detection increased to 12%. The results were in complete agreement with those of TEM ThT-CLSM; both sensitivity and specificity were 100%. Finally, ThT-CLSM results did not vary with section thickness, and small amounts of amyloid could even be detected in 2-μm sections. In conclusion, we found ThT-CLSM to be more sensitive as a screening method for amyloidosis than CR and ThT epifluorescence optical imaging. The method was easier to standardise, provided images with better resolution and resulted in more consistent pathologist diagnoses.



Similar content being viewed by others
References
Westermark P, Benson MD, Buxbaum JN et al (2007) A primer of amyloid nomenclature. Amyloid 14:179–183
Sipe JD, Benson MD, Buxbaum JN et al (2014) Nomenclature 2014: amyloid fibril proteins and clinical classification of the amyloidosis. Amyloid 21:221–224
Kholova I, Niessen HW (2005) Amyloid in cardiovascular system: a review. JClinPathol 58:125–133
Hawkins PN (1995) Amyloidosis. Blood Rev 9:135–142
Le Vine H (1995) Thioflavine T interaction with amyloid β-sheet structures. Amyloid 2:1–6
Naiki H, Higuchi K, Hosokawa M, Takeda T (1989) Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal Biochem 177:244–249
Naiki H, Higuchi K, Matsushima K et al (1990) Fluorometric examination of tissue amyloid fibrils in murine senile amyloidosis: use of the fluorescent indicator, thioflavine T. Lab Investig 62:768–773
Glenner GG (1981) The bases of the staining of amyloid fibers: their physico-chemical nature and the mechanism of their dye–substrate interaction. Prog Histochem Cytochem 13:1–37
Rocken C, Schwotzer EB, Linke P, Saeger W (1996) The classification of amyloid deposits in clinicopathological practice. Histopathology 29:325–335
Linke RP (2012) On typing amyloidosis using immunohistochemistry. Detailed illustrations, review and a note on mass spectrometry. Prog Histochem Cytochem 47(2):61–132
Arbustini E, Verga L, Concardi M, Palladini G, Obici L, Merlini G (2002) Electron and immuno-electron microscopy of abdominal fat identifies and characterizes amyloid fibrils in suspected cardiac amyloidosis. Amyloid 9:108–114
Fernández de Larrea C, Verga L, Morbini P et al (2015) A practical approach to the diagnosis of systemic amyloidoses. Blood 125:2239–2244
Rodriguez FJ, Gamez JD, Vrana JA et al (2008) Immunoglobulin derived depositions in the nervous system: novel mass spectrometry application for protein characterization in formalin-fixed tissues. Lab Investig 88:1024–1037
Theis JD, Dasari S, Vrana JA, Kurtin PJ, Dogan A (2013) Shotgun-proteomics-based clinical testing for diagnosis and classification of amyloidosis. J Mass Spectrom 48:1067–1077
Brambilla F, Lavatelli F, Di Silvestre D, Valentini V, Rossi R, Palladini G, Obici L, Verga L, Mauri P, Merlini G (2012) Reliable typing of systemic amyloidosis through proteomic analysis of subcutaneous adipose tissue. Blood 119(8):1844–1847
Sethi S, Vrana JA, Theis JD, Dogan A (2013) Mass spectrometry based proteomics in the diagnosis of kidney disease. Curr Opin Nephrol Hypertens 22(3):273–280
Picken MM (2015) Proteomics and mass spectrometry in the diagnosis of renal amyloidosis. Clin Kidney J 8(6):665–672
Linke RP (2000) Highly sensitive diagnosis of amyloid and various amyloid syndromes using Congo red fluorescence. Virchows Arch 436:439–448
Linke RP, Gärtner HV, Michels H (1995) High sensitive diagnosis of AA-amyloidosis using Congo red and immunohistochemistry detects missed amyloid deposits. J Histochem Cytochem 9:863–869
Michels H, Linke RP (1998) Clinical benefits of diagnosing incipient AA amyloidosis in pediatric rheumatic diseases as estimated from a retrospective study. Amyloid 5:200–207
Elghetany MT, Saleem A, Barr K (1989) The Congo red stain revisited. Ann Clin Lab Sci 19:190–195
Marcus A, Sadimin E, Richardson M, Goodell L, Fyfe B (2012) Fluorescence microscopy is superior to polarized microscopy for detecting amyloid deposits in Congo red–stained trephine bone marrow biopsy specimens. Am J Clin Pathol 38(4):590–593
Giorgadze TA, Shiina N, Baloch ZW, Tomaszewski JE, Gupta PR (2004) Improved detection of amyloid in fat pad aspiration: an evaluation of Congo red stain by fluorescent microscopy. Diagn Cytopathol 31:300–306
Turillazzi E, Karch SB, Neri M, Pomara C, Riezzo I, Fineschi V (2008) Confocal laser scanning microscopy. Using new technology to answer old questions in forensic investigations. Int J Legal Med 122:173–177
White JG, Amos WB, Fordham M (1987) An evaluation of confocal versus conventional imaging of biological structures by fluorescence light microscopy. J Cell Biol 105:41–48
Amos WB, White JG (2003) How the confocal laser scanning microscope entered biological research. Biol Cell 95:335–342
Sanderson MJ, Smith I, Parker I, Bootman MD (2014) Fluorescence microscopy. Cold Spring Harb Protoc. doi:10.1101/pdb.top071795
Falk RH (2005) Diagnosis and management of the cardiac amyloidoses. Circulation 112:2047–2060
Puchtler H, Sweat F, Levine M (1962) On the binding of Congo red by amyloid. J Histochem Cytochem 10:355–364
Michels J, Buntzow M (2010) Assessment of Congo red as a fluorescence marker for the exoskeleton of small crustaceans and the cuticle of polychaetes. J Microsc 238:95–101
Simel DL, Samsa GP, Matchar DB (1991) Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol 44:763–770
Westermark GT, Johnson KH, Westermark P (1999) Staining methods for identification of amyloid tissue. Methods Enzymol 309:3–25
Elghetany MT, Saleem A (1988) Methods for staining amyloid in tissues: a review. Stain Technol 63:201–212
Khurana R, Uversky VN, Nielsen L, Fink AL (2001) Is Congo red an amyloid-specific dye? J Biol Chem 276:22715–22721
Howie AJ, Brewer DB (2009) Optical properties of amyloid stained by Congo red: history and mechanisms. Micron 40:285–301
Howie AJ, Brewer DB, Howell D, Jones AP (2008) Physical basis of colors seen in Congo red-stained amyloid in polarized light. Lab Investig 88:232–242
Biancalana M, Koide S (2010) Molecular mechanism of thioflavin-T binding to amyloid fibrils. Biochim Biophys Acta 1804:1405–1412
Khurana R, Coleman C, Ionescu-Zanetti C et al (2005) Mechanism of thioflavin T binding to amyloid fibrils. J Struct Biol 151:229–238
Vassar PS, Culling CF (1959) Fluorescent stains with special reference to amyloid and connective tissues. Arch Pathol 68:487–498
Krebs MR, Bromley EH, Donald AM (2005) The binding of thioflavin-T to amyloid fibrils: localisation and implications. J Struct Biol 149:30–47
Scivetti M, Favia G, Fatone L, Maiorano E, Crincoli V (2016) Concomitant use of Congo red staining and confocal laser scanning microscopy to detect amyloidosis in oral biopsy: a clinicopathological study of 16 patients. Ultrastruct Pathol 40:86–91
Acknowledgments
The authors thank Elisabetta Baliello, Alessandra Dubrovich, Luca Braghetto, Daniele Iannazzone and Giulia Fregonese for skillful technical assistance.
Authors’ contributions
CC, MF and AA performed the research; MD and MV performed ME experiments; AA, CC and MF designed the research study; AA contributed essential reagents and tools; CC, MF and ACF analysed the data; CC, MF and AA wrote the paper; AA, MV, FA and GT reviewed the paper.
All authors were involved in writing the manuscript and approving the final submitted version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
University fundings
This work was supported by research grants from the Veneto Region, RF-VEN303/09, and from the University of Padua: CPDR099073, CPDA108809, 60A07-5074, 60A07-8587 and GRIC13IH1H.
Additional information
Chiara Castellani and Marny Fedrigo contributed equally to this work.
Electronic supplementary material
ESM 1
(DOC 33 kb)
Supplementary Figure 1
(JPEG 244 kb)
Supplementary Figure 2
(JPEG 205 kb)
Supplementary Figure 3
(JPEG 328 kb)
Rights and permissions
About this article
Cite this article
Castellani, C., Fedrigo, M., Frigo, A.C. et al. Application of confocal laser scanning microscopy for the diagnosis of amyloidosis. Virchows Arch 470, 455–463 (2017). https://doi.org/10.1007/s00428-017-2081-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-017-2081-7