Abstract
Main conclusion
Plant Biomarkers are objective indicators of a plant’s cellular state in response to abiotic and biotic stress factors. They can be explored in crop breeding and engineering to produce stress-tolerant crop species.
Abstract
Global food production safely and sustainably remains a top priority to feed the ever-growing human population, expected to reach 10 billion by 2050. However, abiotic and biotic stress factors negatively impact food production systems, causing between 70 and 100% reduction in crop yield. Understanding the plant stress responses is critical for developing novel crops that can adapt better to various adverse environmental conditions. Using plant biomarkers as measurable indicators of a plant’s cellular response to external stimuli could serve as early warning signals to detect stresses before severe damage occurs. Plant biomarkers have received considerable attention in the last decade as pre-stress indicators for various economically important food crops. This review discusses some biomarkers associated with abiotic and biotic stress conditions and highlights their importance in developing stress-resilient crops. In addition, we highlighted some factors influencing the expression of biomarkers in crop plants under stress. The information presented in this review would educate plant researchers, breeders, and agronomists on the significance of plant biomarkers in stress biology research, which is essential for improving plant growth and yield toward sustainable food production.
Similar content being viewed by others
Introduction
Food is essential to our daily lives and well-being because it provides energy to power all metabolic processes and nutrients for proper growth and disease resistance (Holder 2019). Food security refers to the condition in which people always have social, economic, and physical access to safe, nutritious, and sufficient food to meet their dietary requirements for a healthy life (Sadati et al. 2021). Ensuring adequate food security for the global human population, projected to expand to 10 billion by 2050, a 34% increase over the current population size, is a paramount global concern and imperative (Boretti and Rosa 2019).
One of the foremost strategies to attain global food security entails a substantial boost in food crop production. A recent study conducted by Galieni et al. (2021) underscores the urgency of this matter, revealing that, given the current population growth rate, food production must surge by approximately 70% to align with existing food demand. Between 2000 and 2019, the total primary crop production recorded a 54% increase, reaching 9.4 billion tonnes (FAO 2022). However, this positive trend does not extend uniformly to developing countries. In stark contrast, food production per capita in Africa has experienced a decline of about 5–13% over the past few decades, with approximately 73 million people suffering from severe food insecurity (Mohamed et al. 2021; Bjornlund et al. 2020). The principal causes of global food insecurity are abiotic and biotic stress factors.
Abiotic factors such as drought, salinity, heavy metal stress, flooding, and extreme temperatures significantly impact crop production and contribute to food insecurity in developed and developing countries (Summy et al. 2020). These stress factors endanger approximately 90% of arable lands, leading to a 70% reduction in major food crops (Waqas et al. 2019). For instance, drought was the primary cause of grain production shortages in the twenty-first century, with approximately one-third of global drought incidents occurring in Sub-Saharan Africa. Ethiopia and Kenya, in particular, endured some of the most severe drought periods in the past four decades (Kogan et al. 2019; Ofori et al. 2021). Furthermore, global temperature will rise by 2–4.9 °C by 2100, and approximately 5 million sites will experience heavy metal contamination at concentrations above regulatory limits (Raftery et al. 2017; Gonzalez Henao and Ghneim-Herrera 2021).
Biotic stress factors affect crop production and food security worldwide (Kaur et al. 2021). These factors, including bacteria, viruses, fungi, nematodes, weeds, and insects, are a huge constraint, destroying about one-third of agricultural produce valued at 750 billion US dollars annually (Mesterházy et al. 2020). According to the Food and Agriculture Organization (FAO) of the United Nations (UN), plant diseases alone incur global damages of 220 billion USD, while uncontrolled weeds could cause a 100% loss in crop yield annually in both developing and developed nations (He and Krainer 2020; Chauhan 2020). Biotic stress factors have historically played a role in some of the most severe famines. For example, in the United States, Puccinia graminis tritici fungi caused an epidemic that resulted in the loss of millions of bushels of wheat (Prasad et al. 2023). Additionally, the cassava mosaic disease epidemic in India, Sri Lanka, and Kenya has resulted in a yearly loss of approximately 25 million tons of cassava, which can lead to famine in subsequent years, especially in countries where it is a staple crop. These multifaceted challenges pose a significant threat to food security on a global scale.
Developing innovative methods and technologies to control or enhance plants' resistance to stress factors has become critical in improving crop growth and yield (Hareesh et al. 2023). An integral component of advancing these methodologies is gaining a profound understanding of plant response patterns to external influences (Galieni et al. 2021). Plants have a dynamic homeostasis system, enabling them to maintain a stable internal state, even amidst unpredictable external conditions. This equilibrium is crucial for their survival and optimal functionality (Torday 2015).
Plants synthesize biomarkers in response to stress to regulate cellular homeostasis. These biomarkers represent specific molecules or compounds that serve as measurable and quantifiable indicators of a plant's reaction to external stimuli (Steinfath et al. 2010). A diverse array of substances, including phytohormones, enzymes, proteins, and nucleic acids, constitute plant biomarkers, serving a pivotal role in monitoring and responding to changes in a plant's environment. Furthermore, they function as precursors, enabling the detection of potential stress well before it manifests as physical symptoms (Alharbi 2020).
Understanding plant physiology and developing strategies to improve crop resilience and productivity in changing environmental conditions. Studying plant biomarkers is an essential aspect of achieving this goal (Zhou et al. 2022). This review presents an overview of the typical cellular biomarkers expressed by plants in response to abiotic and biotic stress factors. It also discusses methods of identifying these biomarkers, their importance in crop engineering, and factors influencing their expression. The information provided in this review would enable agronomists and plant biotechnologists to develop rapid intervention mechanisms to improve crop resilience against both abiotic and biotic stress factors, thus contributing to global food security.
Plant biomarkers
Biomarkers have played a role in modern science for over half a century, but their significance has seen a noticeable increase since the twenty-first century. This surge can be attributed to new technological advancements that have made it possible to generate and validate biomarkers (Rapley and Whitehouse 2015). Biomarkers are indicators of the cellular state of an organism in response to environmental and biological factors (Paniagua-Michel and Olmos-Soto 2016). They are quantifiable and reproducible, and their concentrations differ significantly from those found in normal, unaffected organisms (Bodaghi et al. 2023). Plants, for instance, synthesize biomarkers in response to abiotic and biotic stress factors. These biomarkers function as early warning signals in plants, allowing the detection of stressors before they cause severe damage, often manifested as physical symptoms (Ernst 1999).
There are numerous laboratory-based techniques available for detecting and analyzing biomarkers in plant tissues. These methods involve examining either the biomarker itself or the genes that encode it (Pérez-Clemente et al. 2013). Examples of these techniques include Western blotting, MALDI-TOF, SDS-PAGE, 2D-GE, northern blotting, enzyme-linked immunosorbent assay (ELISA), LC–MS, and polymerase chain reaction (PCR) (Yang et al. 2021). In recent years, omic technologies have provided a more holistic understanding and aided in identifying plant biomarkers indicative of stress conditions. These include techniques such as genomics to identify significant stress-associated genes, proteomics to study variations in protein abundance relative to induced stress, metabolomics to study variations in cellular metabolites in response to stress, and transcriptomics to analyze gene expression patterns (Roychowdhury et al. 2023). Furthermore, the advancement of next-generation sequence approaches such as microarrays, RNA sequencing, and single-molecule real-time sequencing have provided high-throughput, sensitive and rapid methods of generating data from omic techniques (Saeed et al. 2022; Udawat 2023).
While plant biomarkers may not exhibit the same level of specificity as those in mammalian systems, they still play a significant role in detecting and mitigating plant stress factors (Steinfath et al. 2010). Given the increasing impact of abiotic and biotic stressors on plants, there is a growing global interest in using biomarkers at cellular and molecular levels to detect stress early, monitor changes in plant metabolism in response to stress, and prevent irreversible damage (Fernandez et al. 2016; Paes de Melo et al. 2022). This review will explore plant biomarkers with differential expression patterns under stress conditions. These biomarkers include abscisic acid, aquaporin, dehydrin, transcription factors, heat shock proteins, antioxidant enzymes, and sRNA.
Abscisic acid as a hormonal biomarker in plant stress responses
Abscisic acid (ABA) is a sesquiterpenoid with 15 carbon atoms synthesized from β-carotene via either the carotenoid pathway or the indirect pathway (mevalonic acid-independent pathway), as demonstrated in Fig. 1 (Hewage et al. 2020; Chen et al. 2020).
The biosynthesis of Abscisic acid via the direct and indirect pathway. G-3-P—glyceraldehyde-3-phosphate; DXS—Deoxyoxylulose-5-phosphate synthase; MEP-2-C-methyl-d-erythriotl-4-phosphate; IPP—Isopentenyldiphosphate; FPS—Farnesyl diphosphate synthase; FPP—Farnesyl pyrophosphate; GGPS—geranylgeranyl pyrophosphate synthase; GGPP—Geranylgeranyl pyrophosphate; PSY—Phytoene Synthase; ZDS—phytoene desaturase; Z—ISO-z-carotene desaturase; LCYB—lycopene cyclase; BCH—β-carotenoid hydroxylase; ZEP—Zeaxanthin epoxidase; NXS—Neoxanthin synthase; NCED—9-cis-epoxycarotenoid dioxygenase; SDR—Short chain dehydrogenase; A.A.O—Abscisic acid oxidase
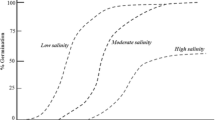
ABA plays a crucial role in the growth and development of plants, acting as an essential phytohormone (Chen et al. 2020). It is especially important in regulating several biochemical, molecular, and physiological processes in crop plants that are exposed to harsh abiotic stress conditions such as drought, salinity, extreme temperature, UV—radiation, and heavy metal stress (Vishwakarma et al. 2017). ABA is a key factor in synchronizing multiple processes that confer stress tolerance, such as root cell elongation, stomata closure, activating transcriptional and post-transcriptional stress defense responses, inducing the expression of stress-related genes, increasing hydraulic conductivity, and metabolic alterations (Muhammad Aslam et al. 2022). Numerous studies have found that an increase in cellular ABA concentrations is linked to adverse environmental conditions (Wang et al. 2021; Hu et al. 2022b; Yang et al. 2023b). For instance, under salt stress, rice plants accumulate ABA, which affects the growth and development of the root meristem (Huang et al. 2021). Similar examples of changes in the cellular concentration of ABA in crop plants under various abiotic stress conditions and their mode of action are reported in Table 1.
In contrast to abiotic stress, the accumulation of ABA during biotic stress can either increase plant tolerance or susceptibility (Gietler et al. 2020). The impact of ABA on plants is also influenced by pathogen type and biotic stress conditions (Bharath et al. 2021; Rasool 2022). High levels of cellular ABA can suppress the immune response of crop plants by repressing salicylic acid activity, making them more susceptible to biotrophic pathogens, such as Magnaporthe oryzae (in barley) and Botrytis cinerea (in tomato), which infect living host cells (Ulferts et al. 2015; Sivakumaran et al. 2016). On the other hand, the accumulation of ABA in crop plants under biotic stress causes stomata closure and increases callose deposits, which protects them from pathogen invasion (Hewage et al. 2020). Table 2 highlights additional examples of changes in cellular ABA concentration in response to biotic stress, including their mechanism of action.
Aquaporin as a biochemical marker in plant stress responses
Aquaporins (AQP) are transmembrane proteins that range in molecular weights from 23 to 31 kDa (Kapilan et al. 2018). They are found in various parts of plants, such as roots, leaves, seeds, flowers, and fruits, under normal physiological and stressful conditions (Hoai et al. 2020; Li et al. 2022)). Given the sedentary nature of plants, their numerous intracellular compartments, and the lack of a specialized circulatory system, there is a critical need for coordinated water regulation to adapt to multiple abiotic stresses, including salinity, drought, temperature, nutrient limitation, and heavy-metal toxicity (Banerjee and Roychoudhury 2020).
AQP serves as a channel for transporting and maintaining cellular water, ion, and neutral solutes, which explains their vital role in regulating some physiological and metabolic processes, such as root/leaf hydraulic conductivity, cell osmoregulation, transpiration, stomatal closure, cell regeneration, and cell elongation in plants (Zupin et al. 2017). Given the importance of AQP in plant cells, they are either upregulated or downregulated in response to stress. This modification of AQP abundance under different stressors helps to regulate osmotic balance (Kapilan et al. 2018).
AQP are typically classified into five subfamilies, namely tonoplast intrinsic protein (TIP), plasma membrane intrinsic proteins (PIP), small basic intrinsic proteins (SIP), X intrinsic proteins (XIP), and nodulin 26-like intrinsic proteins (NIP) (Zargar et al. 2017). These families of proteins perform specific roles within the cell during normal physiological conditions. The SIPs and some NIPs mediate the transportation of solvents within the endoplasmic reticulum, and the TIPs and NIPs mainly participate in the movement of minerals and organic micro-compounds due to their lower permeability to water molecules (Afzal et al. 2016). Conversely, the PIPs and TIPs mainly play a part in reactions during drought, cold, and salinity stress (Maurel et al. 2015).
Notably, plants’ resistance to different stressors is directly proportional to the amount, distribution, and efficiency of aquaporins within the cells (Patel and Mishra 2021). For example, a study by (Lian et al. 2006), showed that 20% polyethene glycol (PEG)-induced water stress enhanced the accumulation of root and leaf plasma membrane intrinsic proteins in two rice cultivars, lowland rice (Oryza sativa L. cv. Xiushui 63) and upland rice (Oryza sativa L. cv. Zhonghan 3). Under drought stress, there was a consistent increase in the expression of the aquaporin gene (PIP1;5) in the leaves and roots of the more drought-tolerant pearl millet (Pennisetum glaucum (L.) R. Br) (Iwuala et al. 2020). Further examples of aquaporin expression under different abiotic stress conditions and their mode of action are shown in Table 3.
Dehydrin as a biochemical marker in plant stress responses
Dehydrins (dehydration-induced proteins) are a type of protein that are highly hydrophilic and thermostable, with molecular weights ranging from 22 to 60 kDa (Arumingtyas and Savitri 2013). These proteins belong to group 2 within the late embryogenesis abundant (LEA) family and are the most extensively studied of the seven groups of LEA proteins due to their crucial role in increasing plant tolerance to abiotic stress (Mertens et al. 2018).
During times of abiotic stress, plant organs accumulate dehydrins within their nucleus, mitochondria, cytoplasm, and membranes (Tiwari and Chakrabarty 2021). Due to their hydrophilic and thermostable properties, these proteins are able to maintain structural flexibility, even binding to membrane proteins during water deficit to prevent protein inactivation and coagulation (Liu et al. 2017). Furthermore, dehydrins’ highly disordered and unstructured nature plays a crucial role in increasing tolerance to abiotic stress by preserving cellular integrity through the formation of hydrogen bonds within the cell membrane via coupled folding (Banerjee and Roychoudhury 2016).
According to (Kalemba et al. 2015), dehydrin accumulation in various cellular compartments, organelles, and membranes in beech (Fagus sylvatica L.) seeds during development and storage prevents cellular damage caused by dehydration. Table 4 shows other examples of dehydrin expression in different crop plants under various abiotic stress conditions.
Dehydrins are grouped structurally into five sub-classes, namely SKn, Kn, YnKn, KnSYn, and YnSKn, based on the presence of conserved sequences (lysine-rich K-segment, unique to all Dehydrins; serine-rich S-segment; and tyrosine-rich Y-segment (Sun et al. 2021b). These conserved regions play essential roles in protecting plants from the adverse effects of osmotic stress. For example, the K-segment binds to cell membrane proteins, protecting them from electrolyte leakage and lipid oxidation. The S-segment is responsible for phosphorylation by the SNF1-related protein kinase, which influences the translocation of dehydrins from the cytosol to the nucleus and binding to calcium ions. However, the precise function of the Y-segment remains unknown (Murray and Graether 2022). Stival and colleagues reported the expression of dehydrin genes in Picea glauca in response to drought. They discovered that dehydrins with N1 K2 and N1 AESK2 sequences were the most receptive to the absence of water (Stival Sena et al. 2018).
Transcription Factors as molecular biomarkers in plant stress responses
As stated by Kabir et al. (2021), transcription factors (TFs) are multifunctional proteins that regulate various plant reactions to stress. They bind to transcription-factor binding sites in the promoter region of a DNA sequence, which then triggers a series of downstream reactions that result in the expression of target genes and the subsequent synthesis of functional proteins relative to stress (Wu et al. 2015). Stress signals perceived by cell wall and membrane receptors are transmitted to transcription factors through intracellular compounds such as reactive oxygen species (ROS), Ca2+, phosphatases and protein kinases. These TFs then regulate or stimulate the expression of responsive genes by binding to their respective cis-element (Shahzad et al. 2021).
Numerous families of transcription factors highly regulate plant defense gene expression in response to abiotic and biotic stress factors. These families include basic leucine zipper (bZIP), AP2/ERF, WRKY, NAC (NAM: no apical meristem, ATAF, CUC: cup-shaped cotyledon), drought-response elements binding proteins (DREB) and myeloblastoma (MYB) (Javed et al. 2020; Hrmova and Hussain 2021). Each of these transcription factor families comprises over 100 and can act as positive or negative regulators to enhance tolerance to the respective stress factor (Hu et al. 2022c).
Several research have reported changes in the expression of TF in crop plants in relation to abiotic stress. For example, (Xiang et al. 2008) reported an increased expression of a member of the bZIP transcription factor family (OsbZIP23) in drought-resistant upland rice genotype IRAT109 (Japonica) exposed to drought and salinity stress, as revealed through Northern-blot analysis. (Rahman et al. 2016) reported that the overexpression of finger millet (Eleusine coracana L.) transcription factor (NAC 67) in rice increased the tolerance of the resulting transgenic rice to drought stress by increasing the relative water content. Similarly, (Wei et al. 2019) demonstrated that overexpressing the GmWRKY54 transcription factor in soybeans conferred drought tolerance by activating target genes in the Ca2+ and abscisic acid signalling pathway. Other examples of TF expression under different abiotic stress conditions and their mode of action are shown in Table 5.
TFs are also crucial in the plants’ adaptation mechanism to biotic stress. For example, during a pathogenic attack, TF promotes the activation of pathogenesis-related protein genes and hypersensitive response, thereby increasing the plants’ resilience to the pathogen (Campos et al. 2022). (Kaushal et al. 2021) also reported the upregulation of NAC, bHLH, and MYB transcription factors in banana cultivars resistant to Fusarium stress. Similarly, López et al. (2021) observed an increased resistance of a Columbia tomato cultivar to Fusarium oxysporum f. sp. lycopersici (Fol) infection due to the upregulation of WRKY transcription factor. Table 6 highlights more examples of TF expression under biotic stress.
Heat shock proteins as a biochemical biomarker in plant stress responses
Heat shock proteins (HSP), commonly known as stress proteins, are expressed by all living organisms, including plants and are widely distributed in cellular compartments and organelles such as the nucleus, endoplasmic reticulum, cytoplasm, and chloroplast (ul Haq et al. 2019; Singh et al. 2019). These proteins can be classified into five families based on their sequence homology and molecular weight: small Hsps (sHsp), Hsp60, Hsp70, Hsp90, and Hsp100 (Li and Liu 2019). Under normal physiological conditions, HSP constitutes between 5 and 10% of the total concentration of cellular proteins, where they play a highly significant role in regulating various growth and developmental processes such as controlling the cell cycle, assembling multi-protein units transporting into and out of subcellular compartments, and controlling protein degradation (Park and Seo 2015). However, their expression significantly increases under abiotic and biotic stress, a crucial adaptation to crop plants' stress tolerance (Hu et al. 2022a).
Initially identified as proteins upregulated in plant cells under heat stress, it is now widely recognized that their expression also increases in response to other abiotic stress, such as heavy metal stress, drought, cold and UV radiation (Jacob et al. 2017). These stress factors induce changes in the physiological, cellular and metabolic function of the cell, leading to the aggregation, misfolding and dysfunction of native and non-native proteins (Mishra et al. 2018). HSPs function as molecular chaperones and perform several protective roles that safeguard the cell from the harmful effects of stress. For example, they buffer and bind to the hydrophobic regions of unfolded polypeptides during translation, preventing aggregation and amino-terminal misfolding and ensuring proper folding of the polypeptide chain (Roy et al. 2019). Additionally, they assist in stabilizing protein structure, maintaining normal conformation, and regulating cellular homeostasis (Khan et al. 2021). Using SDS-PAGE, isoelectric focusing (IEF), western blot, and dot blot techniques, Polenta et al. (2020) discovered an increased expression of HSP in tomatoes in response to extreme heat and cold conditions. Their findings highlight the importance and application of HSP as a plant stress biomarker (Polenta et al. 2020). Table 7 highlights some examples of HSP expression and their mechanism of conferring tolerance to plants under different abiotic stress conditions.
As observed in abiotic stress, HSPs are also integral components of the adaptive strategies employed by plants to mitigate the adverse effects of biotic stress. They enhance tolerance to biotic stress by regulating the stability and accumulation of various stress-responsive proteins, including pathogenesis-related (PR) proteins and antioxidant enzymes, thus detoxifying reactive oxygen species and preserving membrane stability. Numerous studies have documented the differential expression of heat shock proteins (HSP) in response to biotic stress. In a study conducted by Li et al. (2021), it was observed that the increased expression of HSP24 improved the resistance of grape berries (Vitis vinifera Cv ‘Kyoho’) against Botrytis cinerea infection. The researchers reported that this was due to the physical interaction of HSP24 with pathogenesis-related (PR) proteins, leading to the activation of the salicylic acid defense pathway against the fungi. Table 8 shows similar examples of HSP expression under different biotic stress conditions, including their mode of action.
Antioxidant enzymes as biochemical markers in plant stress responses
Enzymatic antioxidants are essential in promoting plant growth and development by counteracting the deteriorating effects of oxidative stress. They break down and eliminate free radicals produced in plant cells during biotic and abiotic stress (Saisanthosh et al. 2018). These antioxidant enzymes include superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), ascorbate peroxidase (APX), glutathione peroxidase (GuPx), and glutathione reductase (GR). SOD catalyzes the conversion of superoxide radicals to O2 and H2O2, CAT converts two molecules of H2O2 into water and O2, and POX scavenges H2O2 within extracellular spaces. In addition, APX utilizes ascorbic acid to reduce H2O2 to water, GPX catalyzes the breakdown of H2O2 and GR catalyzes the conversion of oxidized glutathione (dimeric GSSG) to reduced glutathione (monomeric GSH) (Rajput et al. 2021; Kapoor et al. 2020).
Abiotic stress triggers physiological and metabolic changes such as stomatal closure, reduced CO2 availability, and disruption of photosynthetic enzymes and photosystems (Sachdev et al. 2021). These stress-induced changes causes the accumulation of free radicals and reactive oxygen species such as singlet oxygen, superoxide ion, and hydrogen peroxide in various plant tissues, leading to oxidative damage and cell death (Dumanović et al. 2021). In response to increased ROS production, plants upregulate the synthesis of antioxidant enzymes to scavenge and maintain cellular ROS homeostasis, thereby mitigating the adverse effects of abiotic stress (Huang et al. 2019). Numerous studies have shown that under various abiotic stressors, plants upregulate antioxidant enzymes. Table 9 highlights a few of these.
Biotic stressors, such as pathogenic infections and wounding, trigger specific plant defence responses. This response involves generating elevated levels of reactive oxygen species (ROS), also known as oxidative burst, to prevent pathogen invasion and proliferation and facilitate death (Ali et al. 2018). ROS speeds up cell regeneration and wound healing by preventing pathogen invasion at the injury site (Polaka et al. 2022). Furthermore, ROS acts as a signalling molecule and regulates several signalling pathways involving cell wall modification, changes in gene expression and hypersensitive response (HR), further protecting plants from biotic stress (Lehmann et al. 2015).
Nevertheless, excess production of ROS beyond a specific concentration threshold disrupts cellular homeostasis, resulting in protein peroxidation, enzyme inhibition, breakdown of cellular components, DNA fragmentation, activation of apoptosis, and cell death (Wang et al. 2019b). Plants deploy antioxidant enzymes as the foremost defense line to counteract these detrimental effects. These enzymes play a crucial role in protecting plants from the harmful consequences of ROS generated by biotic stress factors (Sahu et al. 2022). Table 10 highlights examples of enzymatic antioxidants expressed in response to biotic stress factors and their mechanism of action.
Small RNA as a molecular biomarker in plant stress responses
Plant Small RNAs (sRNA) constitute a category of non-coding ribonucleic acid molecules spanning from 21 to 24 nucleotides in length (Morgado and Johannes 2019). They are ubiquitously distributed across diverse cell types and tissues, actively participating in various biological processes, including plant reproduction, growth, and response to biotic and abiotic stressors (Zhan and Meyers 2023; González Plaza 2020). Plant small RNAs (sRNAs) can be classified into several categories based on their biogenesis, including microRNAs (miRNAs), piwi interacting RNAs (piRNAs), small interfering RNAs (siRNAs), small nuclear RNAs (snRNAs), and small nucleolar RNAs (snoRNAs) (Brant and Budak 2018). However, miRNAs and siRNAs are the most widely studied, primarily due to their pivotal roles in enhancing plant resilience against abiotic and biotic stress factors (Chen et al. 2018).
miRNAs and siRNAs are both generated from double-stranded RNAs (dsRNAs) in a series of downstream reactions involving RNA polymerase and Dicer-like (DCL) proteins (Mahto et al. 2020). However, while DCL1 trims miRNA, siRNA is generated from multiple pathways involving diverse exogenous and endogenous dsRNAs precursors by DCL2-4 proteins. The generated miRNAs and siRNAs are then incorporated into Argonaute (AGO) proteins to form the RNA-induced silencing complex (RISC). This complex regulates target genes at the transcription and post-transcriptional levels (Tang et al. 2021).
The mechanism of action of sRNA at target sites involves transcriptional and post-transcriptional gene silencing through DNA methylation, RNA slicing, histone modification, and translational repression (Tang et al. 2022; Patel et al. 2020). Additionally, they exhibit diverse regulatory patterns in response to varying stress conditions, with upregulation observed in positive regulators and downregulation in negative stress regulators (Sun et al. 2021a). While specific sRNAs are conserved, overseeing shared traits across plant species, others are specific to particular species. Both species-specific and conserved sRNAs play a pivotal role in plant stress responses and can be used as plant biomarkers (Jyothsna and Alagu 2022).
sRNA acts as a modulator in response to diverse abiotic stress conditions. They regulate the upregulation or downregulation of target genes in stress-associated pathways at both the transcriptional and post-transcriptional levels (Mondal et al. 2023). For instance, miRNA contributes to enhanced drought tolerance by regulating the expression of drought-responsive genes, transcription factors, and other biomolecules, including proline, dehydrin, and LEA proteins (Saroha et al. 2017). Furthermore, miRNA regulates salinity stress tolerance by modulating ion homeostasis and hormone signalling pathways (Banerjee et al. 2017). More examples of some sRNA identified in various crop plants under abiotic stress, their identification procedures, and their mode of action are highlighted in Table 11.
In addition, small RNA (sRNA) enhances plant tolerance to biotic stress by targeting genes involved in regulating multiple plant immune responses, including pathogen-associated molecular pattern(PAMP)- triggered immunity (PTI) and effector-triggered immunity (ETI) (Brant and Budak 2018). Both PTI and ETI work synergistically to induce various defense mechanisms, such as the accumulation of salicylic acid (SA), callose deposition on the cell wall, hypersensitive response (HR), generation of reactive oxygen species, expression of pathogenesis-related (PR) genes, and cell death at the infection site (Tang et al. 2021). For example, In barley (Hordeum vulgare L.), miRNA was upregulated by the infection of Blumeria graminis f. sp. hordei, a fungus that causes powdery mildew disease. Further experiments suggest that increased miRNA expression confers tolerance by activating a cascade of reactions, leading to disease resistance and cell death signalling (Liu et al. 2014). Table 12 highlights examples of some sRNA identified in various crop plants in response to biotic stress, their identification procedures, and their mode of action.
Structure–activity relationship of biomarkers
Understanding how biomolecules are structured is essential in determining their role in plants under various stress conditions. This is referred to as structure–activity relationships (SAR), which explores the relationship between a molecule's biological activity and its three-dimensional (3D) structure. Understanding the structure and c functional groups of a plant stress biomarker aids in predicting physiological and biochemical function (Šamec et al. 2021). For example, aquaporin is a transmembrane protein with unique characteristics that allow it to transport water in plants during osmotic stress (Wang et al. 2020). Aquaporin comprises 6 segments of alpha-helical hydrophobic protein domains and several NPA (asparagine-proline-alanine). The presence of hydrophobic segments and the formation of hydrogen bonds between water molecules and polypeptide residues enables rapid water transport within plant tissues (Adeoye et al. 2021). Similar examples showcasing the structure–activity relationship of various biomarkers can be found in Table 13.
Recent trends in the application of plant stress biomarkers
Application of plant biomarkers in crop engineering
To achieve sustainable agriculture and produce enough food for the world’s growing population, effective strategies for dealing with extreme conditions such as temperature extremes, pathogen attacks, herbivores, drought, salinity, and heavy metal stress are required (López-Arredondo et al. 2015). To achieve this, it is important to understand the cellular, epigenetic, and molecular mechanisms that orchestrate plant response to various biotic and abiotic stressors. This will lay the groundwork for engineering crops with faster growth rates, higher yield, and productivity (Jiménez Bremont et al. 2013). Modern crop science research has been transformed by the understanding of omic technologies (metabolomics, genomics, proteomics, and transcriptomics), which allow for more robust studies on the primary metabolites, proteins, genes, and molecular networking pathways associated with plant responses to various abiotic and biotic stresses (Yuan et al. 2008). The findings of these studies have aided in the identification of biomarkers that confer resilience to an adverse stress factor, as well as in the introduction of these desired characteristics into model crops and various economically important crops such as barley, maize, wheat, and rice, among others (Yang et al. 2021). These biomarkers are critical in developing new crop varieties (transgenic crops) that are more resistant to adverse environmental conditions (Rodziewicz et al. 2014). Plant biomarkers are widely used in genetic crop engineering to create transgenic crops with higher yields under adverse abiotic and biotic conditions (Leetanasaksakul et al. 2022; Bakhsh and Hussain 2015). Transgenic crops have proven to be a complementary and effective alternative in modern agriculture, increasing yield by 22%, reducing pesticide use by 37%, and increasing profit by 68%. These crops are grown on approximately 180 million hectares worldwide (James 2014).
Researchers have discovered that overexpressing the barley dehydrin gene (HVA1) in wheat can lead to the development of transgenic wheat that is better adapted to salinity and drought stress. The transgenic wheat plants displayed improved membrane stability and reduced electrolyte leakage, according to research by (Habib et al. 2022). The NAC transcription factor has been identified as a critical TF that enhances the resilience of cowpeas (Vigna unguiculata L. Walp.) to a range of environmental stresses, including drought, heat, cold, and salinity. By overexpressing two native cowpea NAC genes (VuNAC1 and VuNAC2), significant improvements in tolerance to these stressors were achieved. The resulting transgenic plants displayed enhanced antioxidant activity, membrane integrity, water use efficiency, and Na + /K + balance. These improvements culminated in an estimated threefold increase in growth and yield, (Srivastava et al. 2023).
Application of plant biomarkers in crop breeding
When faced with challenging environmental or biological circumstances, plants may demonstrate alterations that involve either the activation or suppression of specific biomarkers, including transcription factors, enzymes, osmolytes, hormones, and small RNA (Isah 2019). These biomolecules are known to prevent the destruction of cellular components and restore homeostasis, which is critical for plant growth and development under stress conditions (Ben Rejeb et al. 2014). Figure 2 illustrates some biomarkers expressed by plants under stress conditions. Comparing the expression of these biomarkers provides information about the tolerance level of the plants and is also helpful for studying and analyzing different plant genotypes, species, and cultivars (Chaudhary et al. 2020). The knowledge of plant biomarkers has been used in crop breeding to identify phenotypes or cultivars that are more resilient or susceptible to different abiotic and biotic stress factors and has aided in selecting lines with better traits (Fahimirad and Ghorbanpour 2019; Dikobe et al. 2023). For instance, the molecular and physiological adaptations of different Andean potato genotypes (Tuberosum and Andigena) to drought were assessed by Vasquez-Robinet et al. (2008). The Andigena landraces accumulated more transcription factors, heat shock proteins, and antioxidant genes after 17 days of imposed drought, making them better adapted to drought than the Tuberosum genotype. The expressed biomarkers significantly classified the genotypes based on their tolerance and sensitivity level (Vasquez-Robinet et al. 2008). Similarly, Sathish et al. (2022) investigated the oxidative stress and antioxidant enzyme levels of 12 maize genotypes exposed to 8 days of severe drought stress. The cultivars responded differently to the imposed stress with varying concentrations of MDA and antioxidant enzymes. Based on the data obtained, three genotypes were classified as drought tolerant and others as drought sensitive (Sathish et al. 2022).
Factors influencing the expression of plant biomarkers in crop plants under stress
Plant biomarkers are influenced by several factors including tissue or organ specificity, circadian readings, developmental stage, species, and cultivars, which cause differential expression patterns in crops exposed to the same stress conditions (Fernandez et al. 2016).
Under drought stress, two aquaporin proteins, PIPs and TIPs, were differentially expressed in Brassica napus plant tissues. PIPs and TIPs were downregulated in the root but upregulated in the leaves. Reduced AOP expression in the roots may be associated with the need to prevent water loss from the root due to water deficit. In contrast, increased leaf AQP expression enhances water transportation to regulate normal metabolic functions within the plant (Sonah et al. 2017). Similarly, Yu et al. (2019) observed variations in the expression of HSP in the leaves and roots of cassava plants exposed to drought stress. The leaves showed greater levels of upregulated HSP genes compared to the roots. Transcription factors are other biomarkers with variable expression patterns in different plant tissues. Using qRT-PCR, the expression profile of a specific wheat transcription factor, MYB4, in response to salinity stress, was investigated. The findings revealed increased expression levels in the shoots, whereas a simultaneous reduction in expression was observed in the roots (Sukumaran et al. 2023). Furthermore, the exposure of pepper plants (Capsicum annuum L.) to pathogenic infection resulted in variations in the expression profile of antioxidant enzymes in both the roots and leaves (Zheng et al. 2004).
Data from several research studies has shown that plant biomarkers are differentially expressed at different stages of plant growth when exposed to the same stress condition. Castañeda-Saucedo et al. (2014) reported differences in dehydrin accumulation at the seed filling and pod formation stages of common beans (Phaseolus vulgaris L.) subjected to drought stress. In a similar experiment, Samarah and colleagues observed a more significant dehydrin accumulation in soybean seeds (Glycine max L.) at the maturity stage compared to the developmental stage (Samarah et al. 2006). Furthermore, high-throughput techniques, such as gene profiling and RNA sequencing, were employed to examine the expression of the transcription factor (bZIP) in wheat (Triticum aestium L.) plants exposed to heat stress. The investigation revealed variations in expression across various developmental stages, with the highest expression level observed at day five post-anthesis (Agarwal et al. 2019). Ge and colleagues studied the expression of aquaporin at different stages of germination in Brassica napus plants subjected to cold, salinity and drought stress. The findings revealed an up-regulation of the AQP genes at the germination and early seedling stage but downregulated at the maturity stage (Ge et al. 2014).
The time of the day or circadian changes have also been reported to influence the expression of biomarkers in plants under stress conditions. For instance, the expression of Aquaporin (PIP) was upregulated at dawn and downregulated at dusk in different plants, which may be due to an increase in the rate of transpiration during the day (Hachez et al. 2012; Heinen et al. 2014; Ding et al. 2020). In a recent study conducted by Lu et al. (2021), it was discovered that while cold stress induces the activity of transcription factors (OsDREB1B and OsDREB1C) throughout the day, peak levels were observed during daytime as opposed to nighttime. Similarly, in peach plants (Prunus persica L.), cold stress leads to a more significant induction in the activity of dehydrins (DHN 1 and 3) in the morning (Artlip et al. 2013). In addition, transcriptomic analysis revealed that heat stress response genes such as HSP, antioxidant enzymes and specific TFs are more induced by heat stress in the morning and early afternoon than at other times of the day (Bonnot et al. 2021; Blair et al. 2019; Lai et al. 2012). Abscisic acid is another plant stress hormone controlled by circadian changes, with peak levels observed at specific times during the day in different crop plants (Hotta et al. 2013; Khan et al. 2010).
Cultivar or plant species is another factor influencing the expression of biomarkers subjected to the same stress conditions. Several examples of this have been documented in literature. Under salinity stress, two rice cultivars (Cotaxtla and Tres Ríos) exhibited different NAC transcription factor expression patterns (García-Morales et al. 2014). Variations in the expression profile of dehydrin between two grape species (V. vinifera and V. yeshanensis) in response to drought were also reported by Yang et al. (2012). While there was an upregulation of dehydrin genes in the V. yeshanensis species between 1 and 2 days post-drought imposition, a response was only observed in the other species between 2 and 3 days post-drought treatment. The results of the field survey conducted by Oliveria and colleagues revealed that differential expression of antioxidant enzymes was observed among ten different cultivars of cowpea (Vigna unguiculata L.) under nematode infestation (Meloidogyne incognita) (Oliveira et al. 2012). In addition, transcriptome analysis has revealed variations in the expression of the transcription factors (bHLH family, AP2-ERF, MYB and WRKY) in the cultivars of potato, tomato and spinach when subjected to pathogen attack (Bayoumi et al. 2021; Kandel et al. 2020; Upadhyay et al. 2016).
Conclusion
Abiotic and biotic stressors pose severe challenges to global food security, rendering current crop yield insufficient to meet future global food demand. These stressors have been documented as significant contributors to a substantial decline in crop yields, affecting developed and developing nations. Therefore, we need to focus on developing innovative technologies and methods to produce stress-tolerant, widely-adapted, and high-yielding crops under various stress conditions. One way to achieve this is by understanding plant response patterns to external factors. When exposed to an environmental or biological stress factor, some biomolecules are up or downregulated in plants. Such biomolecules can function as effective plant biomarkers to monitor plant stress response. In this review, we discussed common biomarkers expressed by plants during abiotic and biotic stress conditions and the relationship between their biological activity and three-dimensional (3D) structures. Plant stress biomarkers have a wide range of applications in crop breeding and crop engineering in producing stress-resilient crops with higher yields under adverse conditions. In addition, they can be used for identifying plant cultivars, species, and genotypes with the desired or improved traits. Considering the potential of plant stress biomarkers, more research on the mechanism underlining plant responses to various stress factors and intensive development of analytical platforms and databases are encouraged to standardize plant stress biomarkers for use in breeding novel stress-resistant crop varieties with better yield.
Data availability
Not applicable.
References
AbdElgawad H, Zinta G, Hamed BA, Selim S, Beemster G, Hozzein WN, Wadaan MA, Asard H, Abuelsoud W (2020) Maize roots and shoots show distinct profiles of oxidative stress and antioxidant defense under heavy metal toxicity. Environ Pollut 258:113705
Adeoye A, Odugbemi A, Ajewole T (2021) Structure and function of aquaporins: the membrane water channel proteins. Biointerface Res Appl Chem 12:690–705
Adhikari A, Roy D, Adhikari S, Saha S, Ghosh PK, Shaw AK, Hossain Z (2023) microRNAomic profiling of maize root reveals multifaceted mechanisms to cope with Cr (VI) stress. Plant Physiol Biochem 198:107693
Afzal Z, Howton T, Sun Y, Mukhtar MS (2016) The roles of aquaporins in plant stress responses. J Dev Biol 4(1):9
Agarwal P, Baranwal VK, Khurana P (2019) Genome-wide analysis of bZIP transcription factors in wheat and functional characterization of a TabZIP under abiotic stress. Sci Rep 9(1):4608
Alharbi RA (2020) Proteomics approach and techniques in identification of reliable biomarkers for diseases. Saudi J Biol Sci 27(3):968–974
Ali M, Cheng Z, Ahmad H, Hayat S (2018) Reactive oxygen species (ROS) as defenses against a broad range of plant fungal infections and case study on ROS employed by crops against Verticillium dahliae wilts. J Plant Interact 13(1):353–363
Al-Mokadem AZ, Alnaggar AE-AM, Mancy AG, Sofy AR, Sofy MR, Mohamed AKS, Abou Ghazala MM, El-Zabalawy KM, Salem NF, Elnosary ME (2022) Foliar application of chitosan and phosphorus alleviate the potato virus Y-induced resistance by modulation of the reactive oxygen species, antioxidant defense system activity and gene expression in potato. Agronomy 12(12):3064
Artlip TS, Wisniewski ME, Bassett CL, Norelli JL (2013) CBF gene expression in peach leaf and bark tissues is gated by a circadian clock. Tree Physiol 33(8):866–877
Arumingtyas EL, Savitri ES (2013) Protein profiles and dehydrin accumulation in some soybean varieties (Glycine max L. Merr) in drought stress conditions. Am J Plant Sci 4:134–141
Avelange-Macherel MH, Payet N, Lalanne D, Neveu M, Tolleter D, Burstin J, Macherel D (2015) Variability within a pea core collection of LEAM and HSP 22, two mitochondrial seed proteins involved in stress tolerance. Plant Cell Env 38(7):1299–1311
Bairwa A, Sood S, Bhardwaj V, Rawat S, Tamanna T, Siddappa S, Venkatasalam E, Dipta B, Sharma AK, Kumar A (2023) Identification of genes governing resistance to PCN (Globodera rostochiensis) through transcriptome analysis in Solanum tuberosum. Funct Integr Genom 23(3):242
Bakhsh A, Hussain T (2015) Engineering crop plants against abiotic stress: current achievements and prospects. Emirates J Food Agric 2015:24–39
Banerjee A, Roychoudhury A (2016) Group II late embryogenesis abundant (LEA) proteins: structural and functional aspects in plant abiotic stress. Plant Growth Regul 79(1):1–17
Banerjee A, Roychoudhury A (2020) The role of aquaporins during plant abiotic stress responses. In: Plant life under changing environment. Elsevier, Amsterdam, pp 643–661
Banerjee S, Sirohi A, Ansari AA, Gill SS (2017) Role of small RNAs in abiotic stress responses in plants. Plant Gene 11:180–189
Bayoumi SR, Adss IA, Ghozlan MH, Eid AR (2021) Differential gene expression of two potato cultivars in response to infection with Ralstonia solanacearum. J Adv Agric Res 26(4):424–439
Ben Rejeb I, Pastor V, Mauch-Mani B (2014) Plant responses to simultaneous biotic and abiotic stress: molecular mechanisms. Plants 3(4):458–475
Bharath P, Gahir S, Raghavendra AS (2021) Abscisic acid-induced stomatal closure: an important component of plant defense against abiotic and biotic stress. Front Plant Sci 12:615114
Bjornlund V, Bjornlund H, Van Rooyen AF (2020) Why agricultural production in sub-Saharan Africa remains low compared to the rest of the world—a historical perspective. Int J Water Resour Dev 36(sup1):S20–S53
Blair EJ, Bonnot T, Hummel M, Hay E, Marzolino JM, Quijada IA, Nagel DH (2019) Contribution of time of day and the circadian clock to the heat stress responsive transcriptome in Arabidopsis. Sci Rep 9(1):4814
Boba A, Kostyn K, Kozak B, Wojtasik W, Preisner M, Prescha A, Gola EM, Lysh D, Dudek B, Szopa J (2020) Fusarium oxysporum infection activates the plastidial branch of the terpenoid biosynthesis pathway in flax, leading to increased ABA synthesis. Planta 251(2):1–14
Bodaghi A, Fattahi N, Ramazani A (2023) Biomarkers: promising and valuable tools towards diagnosis, prognosis and treatment of Covid-19 and other diseases. Heliyon 9:2
Bonnot T, Blair EJ, Cordingley SJ, Nagel DH (2021) Circadian coordination of cellular processes and abiotic stress responses. Curr Opin Plant Biol 64:102133
Boretti A, Rosa L (2019) Reassessing the projections of the world water development report. NPJ Clean Water 2(1):1–6
Brant EJ, Budak H (2018) Plant small non-coding RNAs and their roles in biotic stresses. Front Plant Sci 9:1038
Calvo-Polanco M, Sanchez-Romera B, Aroca R (2014) Mild salt stress conditions induce different responses in root hydraulic conductivity of Phaseolus vulgaris over-time. PLoS ONE 9(3):e90631
Campos MD, Félix MdR, Patanita M, Materatski P, Albuquerque A, Ribeiro JA, Varanda C (2022) Defense strategies: the role of transcription factors in tomato–pathogen interaction. Biology 11(2):235
Cao H, Yang Z, Song S, Xue M, Liang G, Li N (2022) Transcriptome analysis reveals genes potentially related to maize resistance to Rhizoctonia solani. Plant Physiol Biochem 193:78–89
Castañeda-Saucedo MC, Córdova-Téllez L, Tapia-Campos E, Delgado-Alvarado A, González-Hernández VA, Santacruz-Varela A, Loza-Tavera H, Garcia-de-Los-Santos G, Vargas-Suárez M (2014) Dehydrins patterns in common bean exposed to drought and watered conditions. Rev Fitotec Mex 37(1):59–68
Chapman K, Marchi-Werle L, Hunt T, Heng-Moss T, Louis J (2018) Abscisic and jasmonic acids contribute to soybean tolerance to the soybean aphid (Aphis glycines Matsumura). Sci Rep 8:15148
Chaudhary R, Baranwal VK, Kumar R, Sircar D, Chauhan H (2019) Genome-wide identification and expression analysis of Hsp70, Hsp90, and Hsp100 heat shock protein genes in barley under stress conditions and reproductive development. Funct Integr Genom 19:1007–1022
Chaudhary S, Devi P, Bhardwaj A, Jha UC, Sharma KD, Prasad PV, Siddique KH, Bindumadhava H, Kumar S, Nayyar H (2020) Identification and characterization of contrasting genotypes/cultivars for developing heat tolerance in agricultural crops: current status and prospects. Front Plant Sci 11:587264
Chauhan BS (2020) Grand challenges in weed management, vol 1. Frontiers Media, Lausanne
Chen C, Zeng Z, Liu Z, Xia R (2018) Small RNAs, emerging regulators critical for the development of horticultural traits. Hortic Res 5:589
Chen K, Li GJ, Bressan RA, Song CP, Zhu JK, Zhao Y (2020) Abscisic acid dynamics, signaling, and functions in plants. J Integr Plant Biol 62(1):25–54
Coelho J, Almeida-Trapp M, Pimentel D, Soares F, Reis P, Rego C, Mithöfer A, Fortes AM (2019) The study of hormonal metabolism of Trincadeira and Syrah cultivars indicates new roles of salicylic acid, jasmonates, ABA and IAA during grape ripening and upon infection with Botrytis cinerea. Plant Sci 283:266–277
Conti V, Cantini C, Romi M, Cesare MM, Parrotta L, Del Duca S, Cai G (2022) Distinct tomato cultivars are characterized by a differential pattern of biochemical responses to drought stress. Int J Mol Sci 23(10):5412
Cui W, Wang S, Han K, Zheng E, Ji M, Chen B, Wang X, Chen J, Yan F (2021) Ferredoxin 1 is downregulated by the accumulation of abscisic acid in an ABI5-dependent manner to facilitate rice stripe virus infection in Nicotiana benthamiana and rice. Plant J 107(4):1183–1197
Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61:651–679
Dąbrowska G, Kata A, Goc A, Szechyńska-Hebda M, Skrzypek E (2007) Characteristics of the plant ascorbate peroxidase family. Acta Biol Cracov Bot 49(1):7–17
Dai YS, Liu D, Guo W, Liu ZX, Zhang X, Shi LL, Zhou DM, Wang LN, Kang K, Wang FZ (2023) Poaceae-specific β-1, 3; 1, 4-d-glucans link jasmonate signalling to OsLecRK1-mediated defence response during rice-brown planthopper interactions. Plant Biotechnol J 21(6):1286–1300
Dikobe T, Masenya K, Manganyi MC (2023) Molecular technologies ending with ‘omics’: the driving force toward sustainable plant production and protection. F1000Research 12:480
Ding L, Milhiet T, Couvreur V, Nelissen H, Meziane A, Parent B, Aesaert S, Van Lijsebettens M, Inzé D, Tardieu F (2020) Modification of the expression of the aquaporin ZmPIP2; 5 affects water relations and plant growth. Plant Physiol 182(4):2154–2165
Du Z, Li J, Zhong Z, Dong M (2014) A proteomic analysis of Arachis hypogaea leaf in responses to enhanced ultraviolet-B radiation. Acta Ecol Sin 34:2589–2598
Dumanović J, Nepovimova E, Natić M, Kuča K, Jaćević V (2021) The significance of reactive oxygen species and antioxidant defense system in plants: a concise overview. Front Plant Sci 11:552969
Ekinci M, Yildirim E, Agar G, Arslan Yuksel E, Aydin M, Ors S, Kul R (2023) Determination of cadmium and/or drought stress effects on some plant phytohormone contents and hormone gene expressions in bean (Phaseolus vulgaris L.). Turk J Agric for 47:402–411. https://doi.org/10.55730/1300-011X.3096
Ernst WH (1999) Biomarkers in plants. In: Biomarkers: a pragmatic basis for remediation of severe pollution in Eastern Europe. Springer, New York, pp 135–151
Fahimirad S, Ghorbanpour M (2019) Omics approaches in developing abiotic stress tolerance in rice (Oryza sativa L.). In: Advances in rice research for abiotic stress tolerance. Elsevier, Hoboken, pp 767–779
FAO (2022) World food and agriculture statistical yearbook 2022. FAO, Rome
Farahani AS, Taghavi M (2016) Changes of antioxidant enzymes of mung bean [Vigna radiata (L.) R. Wilczek] in response to host and non-host bacterial pathogens. J Plant Protect Res 56:95
Feng Z-J, Liu N, Zhang G-W, Niu F-G, Xu S-C, Gong Y-M (2019) Investigation of the AQP family in soybean and the promoter activity of TIP2; 6 in heat stress and hormone responses. Int J Mol Sci 20(2):262
Fernandez O, Urrutia M, Bernillon S, Giauffret C, Tardieu F, Le Gouis J, Langlade N, Charcosset A, Moing A, Gibon Y (2016) Fortune telling: metabolic markers of plant performance. Metabolomics 12(10):1–14
Galieni A, D’Ascenzo N, Stagnari F, Pagnani G, Xie Q, Pisante M (2021) Past and future of plant stress detection: an overview from remote sensing to positron emission tomography. Front Plant Sci 11:1975
Garcı́a-Limones C, Hervás A, Navas-CortésJiménez-Diaz JARM, Tena M (2002) Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp. ciceris. Physiol Mol Plant Pathol 61(6):325–337
García-Morales S, Gómez-Merino FC, Trejo-Téllez LI (2014) NAC transcription factor expression, amino acid concentration and growth of elite rice cultivars upon salt stress. Acta Physiol Plant 36:1927–1936
Ge FW, Tao P, Zhang Y, Wang J (2014) Characterization of AQP gene expressions in Brassica napus during seed germination and in response to abiotic stresses. Biol Plant 58(2):274–282
Ghorbani A, Izadpanah K, Tahmasebi A, Afsharifar A, Moghadam A, Dietzgen RG (2022) Characterization of maize miRNAs responsive to maize Iranian mosaic virus infection. 3 Biotech 12(3):69
Gietler M, Fidler J, Labudda M, Nykiel M (2020) Abscisic acid—enemy or savior in the response of cereals to abiotic and biotic stresses? Int J Mol Sci 21(13):4607
González Plaza JJ (2020) Small RNAs as fundamental players in the transference of information during bacterial infectious diseases. Front Mol Biosci 7:101
Gonzalez-Henao S, Ghneim-Herrera T (2021) Heavy metals in soils and the remediation potential of bacteria associated with the plant microbiome. Front Env Sci 15:9
Grigorova B, Vaseva II, Demirevska K, Feller U (2011) Expression of selected heat shock proteins after individually applied and combined drought and heat stress. Acta Physiol Plant 33:2041–2049
Habib I, Shahzad K, Rauf M, Ahmad M, Alsamadany H, Fahad S, Saeed NA (2022) Dehydrin responsive HVA1 driven inducible gene expression enhanced salt and drought tolerance in wheat. Plant Physiol Biochem 180:124–133
Hachez C, Veselov D, Ye Q, Reinhardt H, Knipfer T, Fricke W, Chaumont F (2012) Short-term control of maize cell and root water permeability through plasma membrane aquaporin isoforms. Plant Cell Environ 35(1):185–198
Hao Y, Hao M, Cui Y, Kong L, Wang H (2022) Genome-wide survey of the dehydrin genes in bread wheat (Triticum aestivum L.) and its relatives: identification, evolution and expression profiling under various abiotic stresses. BMC Genom 23(1):1–18
Hareesh PS, Resmi TR, Sheela MN, Makeshkumar T (2023) Cassava mosaic disease in South and Southeast Asia: current status and prospects. Front Sustain Food Syst 7:1086660
He S, Krainer KMC (2020) Pandemics of people and plants: which is the greater threat to food security? Mol Plant 13(7):933–934
Heckathorn SA, Mueller JK, LaGuidice S, Zhu B, Barrett T, Blair B, Dong Y (2004) Chloroplast small heat-shock proteins protect photosynthesis during heavy metal stress. Am J Bot 91(9):1312–1318
Heinen RB, Bienert GP, Cohen D, Chevalier AS, Uehlein N, Hachez C, Kaldenhoff R, Le Thiec D, Chaumont F (2014) Expression and characterization of plasma membrane aquaporins in stomatal complexes of Zea mays. Plant Mol Biol 86:335–350
Hewage KAH, Yang JF, Wang D, Hao GF, Yang GF, Zhu JK (2020) Chemical manipulation of abscisic acid signaling: a new approach to abiotic and biotic stress management in agriculture. Adv Sci 7(18):2001265
Hoai PT, Tyerman SD, Schnell N, Tucker M, McGaughey SA, Qiu J, Groszmann M, Byrt CS (2020) Deciphering aquaporin regulation and roles in seed biology. J Exp Bot 71(6):1763–1773
Holder MD (2019) The contribution of food consumption to well-being. Ann Nutr Metab 74(2):44–52
Hotta CT, Nishiyama MY Jr, Souza GM (2013) Circadian rhythms of sense and antisense transcription in sugarcane, a highly polyploid crop. PLoS ONE 8(8):e71847
Hrmova M, Hussain SS (2021) Plant transcription factors involved in drought and associated stresses. Int J Mol Sci 22(11):5662
Hu C, Yang J, Qi Z, Wu H, Wang B, Zou F, Mei H, Liu J, Wang W, Liu Q (2022a) Heat shock proteins: biological functions, pathological roles, and therapeutic opportunities. Med Commun 3(3):e161
Hu Dd, Dong S, Zhang J, Zhao B, Ren B, Liu P (2022b) Endogenous hormones improve the salt tolerance of maize (Zea mays L.) by inducing root architecture and ion balance optimizations. J Agron Crop Sci 208(5):662–674
Hu Y, Chen X, Shen X (2022c) Regulatory network established by transcription factors transmits drought stress signals in plant. Stress Biol 2022:1–22
Huang L, Zhang F, Wang W, Zhou Y, Fu B, Li Z (2014) Comparative transcriptome sequencing of tolerant rice introgression line and its parents in response to drought stress. BMC Genom 15(1):1–16
Huang H, Ullah F, Zhou D-X, Yi M, Zhao Y (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 10:800
Huang Y, Zhou J, Li Y, Quan R, Wang J, Huang R, Qin H (2021) Salt stress promotes abscisic acid accumulation to affect cell proliferation and expansion of primary roots in rice. Int J Mol Sci 22(19):10892
Huang J, Shrestha K, Huang Y (2022) Revealing differential expression of phytohormones in sorghum in response to aphid attack using the metabolomics approach. Int J Mol Sci 23(22):13782
Hussain MA, Li S, Gao H, Feng C, Sun P, Sui X, Jing Y, Xu K, Zhou Y, Zhang W (2023) Comparative analysis of physiological variations and genetic architecture for cold stress response in soybean germplasm. Front Plant Sci 13:1095335
Isah T (2019) Stress and defense responses in plant secondary metabolites production. Biol Res 52:1
Ito TM, Rampim MC, Vieira CE, Polido PB, Kaschuk G, de Souza SGH (2015) Modulation of the transcriptional activity of the AP2/ERF family (DREB genes) in orange (Citrus sinensis) leaves subjected to drought stress. Afr J Biotech 14(11):901–909
Iwuala E, Odjegba V, Sharma V, Alam A (2020) Drought stress modulates expression of aquaporin gene and photosynthetic efficiency in Pennisetum glaucum (L.) R. Br. genotypes. Curr Plant Biol 21:100131
Jacob P, Hirt H, Bendahmane A (2017) The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol J 15(4):405–414
Jain S, Chittem K, Brueggeman R, Osorno JM, Richards J, Nelson BD Jr (2016) Comparative transcriptome analysis of resistant and susceptible common bean genotypes in response to soybean cyst nematode infection. PLoS ONE 11(7):e0159338
James C (2014) Global status of commercialized biotech/GM crops. Isaaa Ithaca, New York
Javed T, Shabbir R, Ali A, Afzal I, Zaheer U, Gao S-J (2020) Transcription factors in plant stress responses: challenges and potential for sugarcane improvement. Plants 9(4):491
Jiménez Bremont JF, Rodríguez Kessler M, Liu J-H, Gill SS (2013) Plant stress and biotechnology. Biomed Res Int 2013:170367
Jyothsna S, Alagu M (2022) Role of phasiRNAs in plant-pathogen interactions: molecular perspectives and bioinformatics tools. Physiol Mol Biol Plants 28(5):947–961
Kabir SMT, Hossain MS, Bashar KK, Honi U, Ahmed B, Emdad EM, Alam MM, Haque MS, Islam MS (2021) Genome-wide identification and expression profiling of AP2/ERF superfamily genes under stress conditions in dark jute (Corchorus olitorius L.). Ind Crops Prod 166:113469
Kalemba EM, Bagniewska-Zadworna A, Ratajczak E (2015) Multiple subcellular localizations of dehydrin-like proteins in the embryonic axes of common beech (Fagus sylvatica L.) seeds during maturation and dry storage. J Plant Growth Regul 34(1):137–149
Kallamadi PR, Dandu K, Kirti PB, Rao CM, Thakur SS, Mulpuri S (2018) An insight into powdery mildew–infected, susceptible, resistant, and immune sunflower genotypes. Proteomics 18(16):1700418
Kandel SL, Hulse-Kemp AM, Stoffel K, Koike ST, Shi A, Mou B, Van Deynze A, Klosterman SJ (2020) Transcriptional analyses of differential cultivars during resistant and susceptible interactions with Peronospora effusa, the causal agent of spinach downy mildew. Sci Rep 10(1):6719
Kapilan R, Vaziri M, Zwiazek JJ (2018) Regulation of aquaporins in plants under stress. Biol Res 51(1):1–11
Kapoor D, Bhardwaj S, Landi M, Sharma A, Ramakrishnan M, Sharma A (2020) The impact of drought in plant metabolism: how to exploit tolerance mechanisms to increase crop production. Appl Sci 10(16):5692
Karakus YY (2020) Typical catalases: function and structure. In: Glutathione system and oxidative stress in health and disease. IntechOpen, London, pp 1–16
Kaur R, Choudhury A, Chauhan S, Ghosh A, Tiwari R, Rajam MV (2021) RNA interference and crop protection against biotic stresses. Physiol Mol Biol Plants 27(10):2357–2377
Kaushal M, Mahuku G, Swennen R (2021) Comparative transcriptome and expression profiling of resistant and susceptible banana cultivars during infection by Fusarium oxysporum. Int J Mol Sci 22(6):3002
Khan N, Farzana N (2014) Antioxidant enzymes and protein profiles in wheat seedlings under abiotic stress. Am J Res Com 2(12):155–167
Khan S, Rowe SC, Harmon FG (2010) Research article Coordination of the maize transcriptome by a conserved circadian clock. BMC Plant Biol 10:1
Khan S, Jabeen R, Deeba F, Waheed U, Khanum P, Iqbal N (2021) Heat shock proteins: classification, functions and expressions in plants during environmental stresses. J Bioresourc Manag 8(2):9
Khan W, Khan A, Ullah A, Haq SIU, Hassan N, Iqbal B, Ahmad N, Mahmoud EA, Elansary HO (2023) Insights concerning advancing the agroecological sustainability of salinity tolerance through proteomics profiling of hexaploid wheat (Triticum aestivum L.). S Afr J Bot 158:142–148
Kibria MG, Hossain M, Murata Y, Hoque MA (2017) Antioxidant defense mechanisms of salinity tolerance in rice genotypes. Rice Sci 24(3):155–162
Kim H-S, Park W, Lim H-G, Eom S, Lee J-H, Carlson JE, Ahn S-J (2019) NaCl-induced CsRCI2E and CsRCI2F interact with aquaporin CsPIP2; 1 to reduce water transport in Camelina sativa L. Biochem Biophys Res Commun 513(1):213–218
Kimotho RN, Baillo EH, Zhang Z (2019) Transcription factors involved in abiotic stress responses in Maize (Zea mays L.) and their roles in enhanced productivity in the post genomics era. PeerJ 7:e7211
Koc M, Gökçen I, Odabaşioğlu M, Yildiz K (2017) The effects of drought on the level of isoforms of aquaporin in cv. ‘Horozkarasi’ grapevine. Sci Pap Ser B Hortic 61:256
Kogan F, Guo W, Yang W (2019) Drought and food security prediction from NOAA new generation of operational satellites. Geomat Nat Haz Risk 10(1):651–666
Korayem AM, El-Bassiouny HMS, Abdallah MMS, El-Monem AAA, Mohamed MMM, El-Ashry SM (2022) Physiological and biochemical changes in the wheat plant (Triticum aestivum L.) infected with nematodes. Asian J Plant Sci 21(4):613–628. https://doi.org/10.3923/ajps.2022.613.628
Kosová K, Vítámvás P, Hlaváčková I, Urban M, Vlasáková E, Prášil I (2015) Responses of two barley cultivars differing in their salt tolerance to moderate and high salinities and subsequent recovery. Biol Plant 59(1):106–114
Lai AG, Doherty CJ, Mueller-Roeber B, Kay SA, Schippers JH, Dijkwel PP (2012) CIRCADIAN CLOCK-ASSOCIATED 1 regulates ROS homeostasis and oxidative stress responses. Proc Natl Acad Sci 109(42):17129–17134
Leetanasaksakul K, Roytrakul S, Phaonakrop N, Kittisenachai S, Thaisakun S, Srithuanok N, Sriroth K, Soulard L (2022) Discovery of potential protein biomarkers associated with sugarcane white leaf disease susceptibility using a comparative proteomic approach. PeerJ 10:e12740
Lehmann S, Serrano M, L’Haridon F, Tjamos SE, Metraux J-P (2015) Reactive oxygen species and plant resistance to fungal pathogens. Phytochemistry 112:54–62
Lei J, Mei Y, Jin X, Liu Y, Wang L, Chai S, Cheng X, Yang X (2022a) Identification of miRNAs in response to sweet potato weevil (Cylas formicarius) infection by sRNA sequencing. Genes 13(6):981
Lei P, Qi N, Yan J, Zhu X, Liu X, Xuan Y, Fan H, Chen L, Duan Y, Wang Y (2022b) Genome-wide identification of small interfering RNAs from sRNA libraries constructed from soybean cyst nematode resistant and susceptible cultivars. Gene 832:146557
Li J, Liu X (2019) Genome-wide identification and expression profile analysis of the Hsp20 gene family in Barley (Hordeum vulgare L.). PeerJ 7:e6832
Li C, Cao S, Wang K, Lei C, Ji N, Xu F, Jiang Y, Qiu L, Zheng Y (2021) Heat shock protein HSP24 is involved in the BABA-induced resistance to fungal pathogen in postharvest grapes underlying an NPR1-dependent manner. Front Plant Sci 12:646147
Li Q, Tong T, Jiang W, Cheng J, Deng F, Wu X, Chen Z-H, Ouyang Y, Zeng F (2022) Highly conserved evolution of aquaporin PIPs and TIPs confers their crucial contribution to flowering process in plants. Front Plant Sci 12:2945
Li Y, Liu Y, Gao Z, Wang F, Xu T, Qi M, Liu Y, Li T (2023) MicroRNA162 regulates stomatal conductance in response to low night temperature stress via abscisic acid signaling pathway in tomato. Front Plant Sci 14:1045112
Lian H-L, Yu X, Lane D, Sun W-N, Tang Z-C, Su W-A (2006) Upland rice and lowland rice exhibited different PIP expression under water deficit and ABA treatment. Cell Res 16(7):651–660
Lin B-L, Wang H-J, Wang J-S, Zaharia LI, Abrams SR (2005) Abscisic acid regulation of heterophylly in Marsilea quadrifolia L.: effects of R-(−) and S-(+) isomers. J Exp Bot 56(421):2935–2948
Liu G-T, Wang J-F, Cramer G, Dai Z-W, Duan W, Xu H-G, Wu B-H, Fan P-G, Wang L-J, Li S-H (2012) Transcriptomic analysis of grape (Vitis vinifera L.) leaves during and after recovery from heat stress. BMC Plant Biol 12:1–10
Liu J, Cheng X, Liu D, Xu W, Wise R, Shen Q-H (2014) The miR9863 family regulates distinct Mla alleles in barley to attenuate NLR receptor-triggered disease resistance and cell-death signaling. PLoS Genet 10(12):e1004755
Liu Y, Song Q, Li D, Yang X, Li D (2017) Multifunctional roles of plant dehydrins in response to environmental stresses. Front Plant Sci 8:1018
Liu G-T, Wang B-B, Lecourieux D, Li M-J, Liu M-B, Liu R-Q, Shang B-X, Yin X, Wang L-J, Lecourieux F (2021) Proteomic analysis of early-stage incompatible and compatible interactions between grapevine and P. viticola. Hortic Res 2021:8
López WR, Garcia-Jaramillo DJ, Ceballos-Aguirre N, Castaño-Zapata J, Acuña-Zornosa R, Jovel J (2021) Transcriptional responses to Fusarium oxysporum f. sp. lycopersici (Sacc.) Snyder & Hansen infection in three Colombian tomato cultivars. BMC Plant Biol 21(1):1–14
López-Arredondo D, González-Morales SI, Bello-Bello E, Alejo-Jacuinde G, Herrera L (2015) Engineering food crops to grow in harsh environments. F1000Research 2015:4
Lu X, Song S, Xiao Y, Fan F, Zhou Y, Jia G, Tang W, Peng J (2021) Circadian clock-coordinated response to chilling stress in rice. Environ Exp Bot 185:104398
Mahto BK, Katiyar A, Lenka SK, Bansal KC (2020) Small RNA technology for plant abiotic stress tolerance. In: Plant small RNA. Elsevier, Hoboken, pp 521–541
Maurel C, Boursiac Y, Luu D-T, Santoni V, Shahzad Z, Verdoucq L (2015) Aquaporins in plants. Physiol Rev 95(4):1321–1358
Mena E, Reboledo G, Stewart S, Montesano M, Ponce de Leon I (2023) Comparative analysis of soybean transcriptional profiles reveals defense mechanisms involved in resistance against Diaporthe caulivora. BioRxiv 2023:534358
Mertens J, Aliyu H, Cowan DA (2018) LEA proteins and the evolution of the WHy domain. Appl Environ Microbiol 84(15):e00539-e1518
Mesterházy Á, Oláh J, Popp J (2020) Losses in the grain supply chain: causes and solutions. Sustainability 12(6):2342
Miao G, Qin Y, Guo J, Zhang Q, Bao Y (2021) Transcriptome characterization and expression profile of Coix lacryma-jobi L. in response to drought. PLoS ONE 16(9):e0256875
Mishra D, Shekhar S, Singh D, Chakraborty S, Chakraborty N (2018) Heat shock proteins and abiotic stress tolerance in plants. Regul Heat Shock Protein Responses 2018:41–69
Mishra N, Tripathi M, Tripathi N, Tiwari S, Gupta N, Sharma A, Shrivastav M (2021) Changes in biochemical and antioxidant enzymes activities play significant role in drought tolerance in soybean. Int J Agric Technol 17:1425–1446
Mittal M, Dhingra A, Dawar P, Payton P, Rock CD (2023) The role of microRNAs in responses to drought and heat stress in peanut (Arachis hypogaea). The Plant Genome 2023:e20350
Mohamed EMA, Abdallah SMA, Ahmadi A, Lucero-Prisno DE III (2021) Food security and COVID-19 in Africa: implications and recommendations. Am J Trop Med Hyg 104(5):1613
Mondal R, Das A, Bandyopadhyay A (2023) Implications of small RNAs in plant development, abiotic stress response and crop improvement in changing climate. The Nucleus 2023:1–19
Morgado L, Johannes F (2019) Computational tools for plant small RNA detection and categorization. Brief Bioinform 20(4):1181–1192
Motallebi-Azar A, Papp I, Szego A (2019) Dehydrin profiles of some Iranian melon varieties (Cucumis melo L. Merr) under drought stress conditions. Acta Scient Polonor Hort Cult 18(6):75
Mueth NA, Hulbert SH (2022) Small RNAs target native and cross-kingdom transcripts on both sides of the wheat stripe rust interaction. Genomics 114(6):110526
Muhammad Aslam M, Waseem M, Jakada BH, Okal EJ, Lei Z, Saqib HSA, Yuan W, Xu W, Zhang Q (2022) Mechanisms of abscisic acid-mediated drought stress responses in plants. Int J Mol Sci 23(3):1084
Murray MR, Graether SP (2022) Physiological, structural, and functional insights into the cryoprotection of membranes by the dehydrins. Front Plant Sci 2022:13
Ofori SA, Cobbina SJ, Obiri S (2021) Climate change, land, water, and food security: perspectives From Sub-Saharan Africa. Front Sustain Food Syst 5:680924
Ohnishi T, Sugahara S, Yamada T, Kikuchi K, Yoshiba Y, Hirano H-Y, Tsutsumi N (2005) OsNAC6, a member of the NAC gene family, is induced by various stresses in rice. Genes Genet Syst 80(2):135–139
Oliveira JTAd, Andrade N, Martins-Miranda A, Soares A, Gondim D, Araújo-Filho JHd, Freire-Filho F, Vasconcelos I (2012) Differential expression of antioxidant enzymes and PR-proteins in compatible and incompatible interactions of cowpea (Vigna unguiculata) and the root-knot nematode Meloidogyne incognita. Plant Physiol Biochem 51:145–152
Paes de Melo B, Carpinetti PdA, Fraga OT, Rodrigues-Silva PL, Fioresi VS, de Camargos LF, Ferreira MFdS (2022) Abiotic stresses in plants and their markers: a practice view of plant stress responses and programmed cell death mechanisms. Plants 11(9):1100
Paniagua-Michel J, Olmos-Soto J (2016) Modern approaches into biochemical and molecular biomarkers: key roles in environmental biotechnology. J Biotechnol Biomater 6(216):2
Park C-J, Seo Y-S (2015) Heat shock proteins: a review of the molecular chaperones for plant immunity. Plant Pathol J 31(4):323
Park S-H, Lee B-R, La VH, Mamun MA, Bae D-W, Kim T-H (2021) Drought intensity-responsive salicylic acid and abscisic acid crosstalk with the sugar signaling and metabolic pathway in Brassica napus. Plants 10(3):610
Parmar S, Gharat SA, Tagirasa R, Chandra T, Behera L, Dash SK, Shaw BP (2020) Identification and expression analysis of miRNAs and elucidation of their role in salt tolerance in rice varieties susceptible and tolerant to salinity. PLoS ONE 15(4):e0230958
Patel J, Mishra A (2021) Plant aquaporins alleviate drought tolerance in plants by modulating cellular biochemistry, root-architecture, and photosynthesis. Physiol Plant 172(2):1030–1044
Patel A, Tiwari S, Singh M, Prasad SM (2020) Role of sRNAs in abiotic stress tolerance. In: Plant life under changing environment. Elsevier,New York, pp 467–480
Pérez-Clemente RM, Vives V, Zandalinas SI, López-Climent MF, Muñoz V, Gómez-Cadenas A (2013) Biotechnological approaches to study plant responses to stress. Biomed Res Int 2013:654120. https://doi.org/10.1155/2013/654120
Polaka S, Katare P, Pawar B, Vasdev N, Gupta T, Rajpoot K, Sengupta P, Tekade RK (2022) Emerging ROS-modulating technologies for augmentation of the wound healing process. ACS Omega 7(35):30657–30672
Polenta GA, Guidi SM, Ambrosi V, Denoya GI (2020) Comparison of different analytical methods to evaluate the heat shock protein (HSP) response in fruits. Application to tomatoes subjected to stress treatments. Curr Res Food Sci 3:329–338
Pooam M, El-Ballat EM, Jourdan N, Ali HM, Hano C, Ahmad M, El-Esawi MA (2023) SNAC3 transcription factor enhances arsenic stress tolerance and grain yield in rice (Oryza sativa L.) through regulating physio-biochemical mechanisms, stress-responsive genes, and cryptochrome 1b. Plants 12(14):2731
Prasad P, Thakur R, Bhardwaj SC, Savadi S, Gangwar OP, Lata C, Adhikari S, Kumar S, Kundu S, Manjul AS, Prakasha TL, Navathe S, Hegde GM, Game BC, Mishra KK, Khan H, Gupta V, Mishra CN, Kumar S, Kumar S, Singh G (2023) Virulence and genetic analysis of Puccinia graminis tritici in the Indian sub-continent from 2016 to 2022 and evaluation of wheat varieties for stem rust resistance. Front Plant Sci 2023:14. https://doi.org/10.3389/fpls.2023.1196808
Raftery AE, Zimmer A, Frierson DM, Startz R, Liu P (2017) Less than 2 C warming by 2100 unlikely. Nat Clim Chang 7(9):637–641
Rahman H, Ramanathan V, Nallathambi J, Duraialagaraja S, Muthurajan R (2016) Over-expression of a NAC 67 transcription factor from finger millet (Eleusine coracana L.) confers tolerance against salinity and drought stress in rice. BMC Biotechnol 16:7–20
Rai GK, Parveen A, Jamwal G, Basu U, Kumar RR, Rai PK, Sharma JP, Alalawy AI, Al-Duais MA, Hossain MA (2021) Leaf proteome response to drought stress and antioxidant potential in tomato (Solanum lycopersicum L.). Atmosphere 12(8):1021
Rajput VD, Singh RK, Verma KK, Sharma L, Quiroz-Figueroa FR, Meena M, Gour VS, Minkina T, Sushkova S, Mandzhieva S (2021) Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 10(4):267
Rapley R, Whitehouse D (2015) Molecular biology and biotechnology. Royal Society of Chemistry, New York
Rasool N (2022) Plant hormones: role in alleviating biotic stress. Plant Hormones Recent Adv New Perspect Appl 2022:17
Rodziewicz P, Swarcewicz B, Chmielewska K, Wojakowska A, Stobiecki M (2014) Influence of abiotic stresses on plant proteome and metabolome changes. Acta Physiol Plant 36(1):1–19
Rorat T (2006) Plant dehydrins—tissue location, structure and function. Cell Mol Biol Lett 11(4):536–556
Roy S, Mishra M, Dhankher OP, Singla-Pareek SL, Pareek A (2019) Molecular chaperones: key players of abiotic stress response in plants. Genet Enhancement Crops Tolerance Abiotic Stress Mechan Approach I:125–165
Roychowdhury R, Das SP, Gupta A, Parihar P, Chandrasekhar K, Sarker U, Kumar A, Ramrao DP, Sudhakar C (2023) Multi-omics pipeline and omics-integration approach to decipher plant’s abiotic stress tolerance responses. Genes 14(6):1281
Sachdev S, Ansari SA, Ansari MI, Fujita M, Hasanuzzaman M (2021) Abiotic stress and reactive oxygen species: generation, signaling, and defense mechanisms. Antioxidants 10(2):277
Sadati AK, Nayedar M, Zartash L, Falakodin Z (2021) Challenges for food security and safety: a qualitative study in an agriculture supply chain company in Iran. Agric Food Secur 10(1):1–7
Saeed F, Chaudhry UK, Bakhsh A, Raza A, Saeed Y, Bohra A, Varshney RK (2022) Moving beyond DNA sequence to improve plant stress responses. Front Genet 13:874648
Sahu PK, Jayalakshmi K, Tilgam J, Gupta A, Nagaraju Y, Kumar A, Hamid S, Singh HV, Minkina T, Rajput VD (2022) ROS generated from biotic stress: effects on plants and alleviation by endophytic microbes. Front Plant Sci 13:1042936
Saisanthosh K, Sumalatha G, Shuba A, Komala N, Biradar Patil N (2018) Role of enzymatic antioxidants defense system in seeds. Int J Curr Microbiol App Sci 7(1):584–594
Samarah N, Mullen R, Cianzio S, Scott P (2006) Dehydrin-like proteins in soybean seeds in response to drought stress during seed filling. Crop Sci 46(5):2141–2150
Šamec D, Karalija E, Šola I, Vujčić Bok V, Salopek-Sondi B (2021) The role of polyphenols in abiotic stress response: the influence of molecular structure. Plants 10(1):118
Saroha M, Singroha G, Sharma M, Mehta G, Gupta OP, Sharma P (2017) sRNA and epigenetic mediated abiotic stress tolerance in plants. Indian J Plant Physiol 22(4):458–469
Sathish P, Vanaja M, Jyothi-Lakshmi N, Sarkar B, Vijay-Kumar G, Vagheera P, Mohan C, Maheswari M (2022) Impact of water deficit stress on traits influencing the drought tolerance and yield of maize (Zea mays L.) genotypes. Plant Physiol Rep 27(1):109–118
Sau AK, Dhillon MK, Trivedi N (2022) Activation of antioxidant defense in maize in response to attack by Sesamia inferens (Walker). Phytoparasitica 50(5):1043–1058
Shahzad R, Jamil S, Ahmad S, Nisar A, Amina Z, Saleem S, Iqbal MZ, Atif RM, Wang X (2021) Harnessing the potential of plant transcription factors in developing climate resilient crops to improve global food security: current and future perspectives. Saudi J Biol Sci 28(4):2323–2341
Shi J, Zai W, Xiong Z, Wang K, Shui D, Zg J (2023) Small RNA profiling reveals a role of miRNAs in response to Ralstonia solanacearum infection in tomato. J Plant Growth Regul 42(6):3342–3355
Shu Y, Zhang W, Tang L, Li Z, Liu X, Liu X, Liu W, Li G, Ying J, Huang J (2023) ABF1 positively regulates rice chilling tolerance via inducing trehalose biosynthesis. Int J Mol Sci 24(13):11082
Singh RK, Gupta V, Prasad M (2019) Plant molecular chaperones: structural organization and their roles in abiotic stress tolerance. Mol Plant Abiotic Stress Biol Biotechnol 2019:221–239
Sivakumaran A, Akinyemi A, Mandon J, Cristescu SM, Hall MA, Harren FJ, Mur LA (2016) ABA suppresses Botrytis cinerea elicited NO production in tomato to influence H2O2 generation and increase host susceptibility. Front Plant Sci 7:709
Smith MA, Graether SP (2022) The disordered dehydrin and its role in plant protection: a biochemical perspective. Biomolecules 12(2):294
Sonah H, Deshmukh RK, Labbé C, Bélanger RR (2017) Analysis of aquaporins in Brassicaceae species reveals high-level of conservation and dynamic role against biotic and abiotic stress in canola. Sci Rep 7(1):2771
Srivastava R, Kobayashi Y, Koyama H, Sahoo L (2023) Cowpea NAC1/NAC2 transcription factors improve growth and tolerance to drought and heat in transgenic cowpea through combined activation of photosynthetic and antioxidant mechanisms. J Integr Plant Biol 65(1):25–44
Steinfath M, Strehmel N, Peters R, Schauer N, Groth D, Hummel J, Steup M, Selbig J, Kopka J, Geigenberger P (2010) Discovering plant metabolic biomarkers for phenotype prediction using an untargeted approach. Plant Biotechnol J 8(8):900–911
Stephenie S, Chang YP, Gnanasekaran A, Esa NM, Gnanaraj C (2020) An insight on superoxide dismutase (SOD) from plants for mammalian health enhancement. J Funct Foods 68:103917
Stival Sena J, Giguère I, Rigault P, Bousquet J, Mackay J (2018) Expansion of the dehydrin gene family in the Pinaceae is associated with considerable structural diversity and drought-responsive expression. Tree Physiol 38(3):442–456
Sukumaran S, Lethin J, Liu X, Pelc J, Zeng P, Hassan S, Aronsson H (2023) Genome-wide analysis of MYB transcription factors in the wheat genome and their roles in salt stress response. Cells 12(10):1431
Summy Y, Payal M, Akanksha D, Akdasbanu V, Disha P, Mohini P (2020) Effect of abiotic stress on crops. In: Mirza H, Marcelo Carvalho Minhoto Teixeira F, Masayuki F, Thiago Assis Rodrigues N (eds) Sustainable crop production. IntechOpen, Rijeka, p 1. https://doi.org/10.5772/intechopen.88434
Sun C, Ali K, Yan K, Fiaz S, Dormatey R, Bi Z, Bai J (2021a) Exploration of epigenetics for improvement of drought and other stress resistance in crops: a review. Plants 10(6):1226
Sun Z, Li S, Chen W, Zhang J, Zhang L, Sun W, Wang Z (2021b) Plant dehydrins: expression, regulatory networks, and protective roles in plants challenged by abiotic stress. Int J Mol Sci 22(23):12619
Sun J, Wang H, Ren H, Zhao B, Zhang J, Ren B, Liu P (2023) Maize (Zea mays L.) responses to heat stress: mechanisms that disrupt the development and hormone balance of tassels and pollen. J Agron Crop Sci 209:502
Szabala BM, Fudali S, Rorat T (2014) Accumulation of acidic SK 3 dehydrins in phloem cells of cold-and drought-stressed plants of the Solanaceae. Planta 239:847–863
Tang J, Gu X, Liu J, He Z (2021) Roles of small RNAs in crop disease resistance. Stress Biol 1(1):6
Tang Y, Yan X, Gu C, Yuan X (2022) Biogenesis, trafficking, and function of small RNAs in plants. Front Plant Sci 13:825477
Tiwari P, Chakrabarty D (2021) Dehydrin in the past four decades: from chaperones to transcription co-regulators in regulating abiotic stress response. Curr Res Biotechnol 3:249–259
Torday JS (2015) Homeostasis as the mechanism of evolution. Biology 4(3):573–590
Udawat P (2023) Recent advancements in legumes: next generation sequencing and omics approaches. Indian J Agric Sci 93(5):467–474. https://doi.org/10.56093/ijas.v93i5.119566
ul Haq S, Khan A, Ali M, Khattak AM, Gai W-X, Zhang H-X, Wei A-M, Gong Z-H (2019) Heat shock proteins: dynamic biomolecules to counter plant biotic and abiotic stresses. Int J Mol Sci 20(21):5321
Ulferts S, Delventhal R, Splivallo R, Karlovsky P, Schaffrath U (2015) Abscisic acid negatively interferes with basal defence of barley against Magnaporthe oryzae. BMC Plant Biol 15(1):1–13
Upadhyay P, Ganie SH, Rai A, Singh M, Sinha B (2016) Identification of transcription factors in tomato, potentially related to early blight resistance at invasion in host tissue, using microarray expression profiling. S Afr J Bot 106:165–173
Vasquez-Robinet C, Mane SP, Ulanov AV, Watkinson JI, Stromberg VK, De Koeyer D, Schafleitner R, Willmot DB, Bonierbale M, Bohnert HJ (2008) Physiological and molecular adaptations to drought in Andean potato genotypes. J Exp Bot 59(8):2109–2123
Verma RK, Chetia SK, Sharma V, Devi K, Kumar A, Modi MK (2022) Identification and characterization of genes for drought tolerance in upland rice cultivar ‘Banglami’of North East India. Mol Biol Rep 49(12):11547–11555
Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay R, Pandey M (2017) Abscisic acid signaling and abiotic stress tolerance in plants: a review on current knowledge and future prospects. Front Plant Sci 8:161
Wang F, Guo Z, Li H, Wang M, Onac E, Zhou J, Xia X, Shi K, Yu J, Zhou Y (2016) Phytochrome A and B function antagonistically to regulate cold tolerance via abscisic acid-dependent jasmonate signaling. Plant Physiol 170(1):459–471
Wang Q, Lin F, Wei S, Meng X, Yin Z, Guo Y, Yang G (2019a) Effects of drought stress on endogenous hormones and osmotic regulatory substances of common bean (Phaseolus vulgaris L.) at seedling stage. Appl Ecol Environ Res 17:4447–4457
Wang X, Liu H, Yu F, Hu B, Jia Y, Sha H, Zhao H (2019b) Differential activity of the antioxidant defence system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. Sci Rep 9(1):1–11
Wang Y, Zhao Z, Liu F, Sun L, Hao F (2020) Versatile roles of aquaporins in plant growth and development. Int J Mol Sci 21(24):9485
Wang X, Li Q, Xie J, Huang M, Cai J, Zhou Q, Dai T, Jiang D (2021) Abscisic acid and jasmonic acid are involved in drought priming-induced tolerance to drought in wheat. Crop J 9(1):120–132
Wang X, Zhao S, Zhou R, Liu Y, Guo L, Hu H (2023a) Identification of Vitis vinifera MYB transcription factors and their response against grapevine berry inner necrosis virus. BMC Plant Biol 23(1):279
Wang Y, Li H, Zhao C, Yang C, Xu Q, Yuan H, Yang H, Zeng X (2023b) Identification of a novel transcription factor under long-term drought resistance in highland barley: a DNA affinity purification sequencing-based transcriptomic analysis. Chem Biol Technol Agric 10(1):1–11
Waqas MA, Kaya C, Riaz A, Farooq M, Nawaz I, Wilkes A, Li Y (2019) Potential mechanisms of abiotic stress tolerance in crop plants induced by thiourea. Front Plant Sci 10:1336
Wei W, Liang DW, Bian XH, Shen M, Xiao JH, Zhang WK, Ma B, Lin Q, Lv J, Chen X (2019) GmWRKY54 improves drought tolerance through activating genes in abscisic acid and Ca2+ signaling pathways in transgenic soybean. Plant J 100(2):384–398
Wu J, Zhang Z, Zhang Q, Liu Y, Zhu B, Cao J, Li Z, Han L, Jia J, Zhao G (2015) Generation of wheat transcription factor FOX rice lines and systematic screening for salt and osmotic stress tolerance. PLoS ONE 10(7):e0132314
Xiang Y, Tang N, Du H, Ye H, Xiong L (2008) Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant Physiol 148(4):1938–1952
Xu X, Fang P, Zhang H, Chi C, Song L, Xia X, Shi K, Zhou Y, Zhou J, Yu J (2019) Strigolactones positively regulate defense against root-knot nematodes in tomato. J Exp Bot 70(4):1325–1337
Yang Y, He M, Zhu Z, Li S, Xu Y, Zhang C, Singer SD, Wang Y (2012) Identification of the dehydrin gene family from grapevine species and analysis of their responsiveness to various forms of abiotic and biotic stress. BMC Plant Biol 12:1–17
Yang Y, Saand MA, Huang L, Abdelaal WB, Zhang J, Wu Y, Li J, Sirohi MH, Wang F (2021) Applications of multi-omics technologies for crop improvement. Front Plant Sci 12:563953
Yang J-W, Park S-U, Lee H-U, Nam KJ, Lee K-L, Lee JJ, Kim JH, Kwak S-S, Kim HS, Kim Y-H (2023a) Differential responses of antioxidant enzymes and lignin metabolism in susceptible and resistant sweetpotato cultivars during root-knot nematode infection. Antioxidants 12(6):1164
Yang X, Liu C, Li M, Li Y, Yan Z, Feng G, Liu D (2023b) Integrated transcriptomics and metabolomics analysis reveals key regulatory network that response to cold stress in common Bean (Phaseolus vulgaris L.). BMC Plant Biol 23(1):85
Yao L, Yang B, Ma X, Wang S, Guan Z, Wang B, Jiang Y (2020) A genome-wide view of transcriptional responses during Aphis glycines infestation in Soybean. Int J Mol Sci 21(15):5191
You YJ, Ahn SY, Yun HK (2022) Heat shock transcriptional factors (HSFs) are expressed in response to hydrogen peroxide production in grapevines inoculated with Colletotrichum species. Hortic Environ Biotechnol 63(5):735–745
Yu X, Peng YH, Zhang MH, Shao YJ, Su WA, Tang ZC (2006) Water relations and an expression analysis of plasma membrane intrinsic proteins in sensitive and tolerant rice during chilling and recovery. Cell Res 16(6):599–608
Yu X-Y, Yao Y, Hong Y-H, Hou P-Y, Li C-X, Xia Z-Q, Geng M-T, Chen Y-H (2019) Differential expression of the Hsf family in cassava under biotic and abiotic stresses. Genome 62(8):563–569
Yuan JS, Galbraith DW, Dai SY, Griffin P, Stewart CN Jr (2008) Plant systems biology comes of age. Trends Plant Sci 13(4):165–171
Zargar SM, Nagar P, Deshmukh R, Nazir M, Wani AA, Masoodi KZ, Agrawal GK, Rakwal R (2017) Aquaporins as potential drought tolerance inducing proteins: towards instigating stress tolerance. J Proteom 169:233–238
Zeeshan M, Hu YX, Guo XH, Sun CY, Salam A, Ahmad S, Muhammad I, Nasar J, Jahan MS, Fahad S (2023) Physiological and transcriptomic study reveal SeNPs-mediated AsIII stress detoxification mechanisms involved modulation of antioxidants, metal transporters, and transcription factors in Glycine max L.(Merr.) roots. Env Pollut 317:120637
Zhan J, Meyers BC (2023) Plant small RNAs: their biogenesis, regulatory roles, and functions. Annu Rev Plant Biol 74:21–51
Zhang X, Wang W, Wang M, Zhang H-Y, Liu J-H (2016) The miR396b of Poncirus trifoliata functions in cold tolerance by regulating ACC oxidase gene expression and modulating ethylene–polyamine homeostasis. Plant Cell Physiol 57(9):1865–1878
Zheng H-Z, Kim Y-W, Lee H-J, Park R-D, Jung W-J, Kim Y-C, Lee S-H, Kim T-H, Kim K-Y (2004) Quantitative changes of PR proteins and antioxidative enzymes in response to Glomus intraradices and Phytophthora capsici in pepper (Capsicum annuum L.) plants. J Microbiol Biotechnol 14(3):553–562
Zhou R, Jiang F, Niu L, Song X, Yu L, Yang Y, Wu Z (2022) Increase crop resilience to heat stress using omic strategies. Front Plant Sci 13:891861
Zupin M, Sedlar A, Kidrič M, Meglič V (2017) Drought-induced expression of aquaporin genes in leaves of two common bean cultivars differing in tolerance to drought stress. J Plant Res 130(4):735–745
Funding
Open access funding provided by University of the Western Cape.
Author information
Authors and Affiliations
Contributions
OA, MK, and AK designed and conceptualized the manuscript. OA wrote the first draft of the manuscript. OB, MK, and AK reviewed and edited the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest. All authors have read, reviewed, and agreed to submit the review article to Planta.
Additional information
Communicated by Gerhard Leubner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aina, O., Bakare, O.O., Fadaka, A.O. et al. Plant biomarkers as early detection tools in stress management in food crops: a review. Planta 259, 60 (2024). https://doi.org/10.1007/s00425-024-04333-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-024-04333-1