Abstract
Main conclusion
The local and long-distance signaling pathways mediated by the leucine-rich repeat receptor kinase HAR1 suppress root branching and promote primary root length in response to nitrate supply.
Abstract
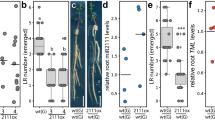
The root morphology of higher plants changes plastically to effectively absorb nutrients and water from the soil. In particular, legumes develop root organ nodules, in which symbiotic rhizobia fix atmospheric nitrogen in nitrogen-poor environments. The number of nodules formed in roots is negatively regulated by a long-distance signaling pathway that travels through shoots called autoregulation of nodulation (AON). In the model plant Lotus japonicus, defects in AON genes, such as a leucine-rich repeat receptor kinase HYPERNODULATION ABERRANT ROOT FORMATION 1 (HAR1), an orthologue of CLAVATA1, and the F-box protein TOO MUCH LOVE (TML), induce the formation of an excess number of nodules. The loss-of-function mutant of HAR1 exhibits a short and bushy root phenotype in the absence of rhizobia. We show that the har1 mutant exhibits high nitrate sensitivity during root development. The uninfected har1 mutant significantly increased lateral root number and reduced primary root length in the presence of 3 mM nitrate, compared with the wild-type and tml mutant. Grafting experiments indicated that local and long-distance signaling pathways via root- and shoot-acting HAR1 additively regulated root morphology under the moderate nitrate concentrations. These findings allow us to propose that HAR1-mediated signaling pathways control the root system architecture by suppressing lateral root branching and promoting primary root elongation in response to nitrate availability.




Similar content being viewed by others
Data availability statement
The data that support the findings of this study are available from the corresponding author, MK, upon reasonable request.
Abbreviations
- AON:
-
Autoregulation of nodulation
- CLE:
-
CLAVATA 3 (CLV3)/EMBRYO SURROUNDING REGION-related
- CLV:
-
CLAVATA
- LR:
-
Lateral root
- LRR-RK:
-
Leucine-rich repeat receptor kinase
- N:
-
Nitrogen
- PR:
-
Primary root
- RSA:
-
Root system architecture
- TML:
-
TOO MUCH LOVE
References
Araya T, Miyamoto M, Wibowo J, Suzuki A, Kojima S, Tsuchiya YN, Sawa S, Fukuda H, von Wirén N, Takahashi H (2014a) CLE-CLAVATA1 peptide-receptor signaling module regulates the expansion of plant root systems in a nitrogen-dependent manner. Proc Natl Acad Sci USA 111(5):2029–2034. https://doi.org/10.1073/pnas.1319953111
Araya T, von Wirén N, Takahashi H (2014b) CLE peptides regulate lateral root development in response to nitrogen nutritional status of plants. Plant Signal Behav 9(7):e29302. https://doi.org/10.4161/psb.29302
Asim M, Ullah Z, Xu F, An L, Aluko O, Wang Q, Liu H (2020) Nitrate signaling, functions, and regulation of root system architecture: insights from Arabidopsis thaliana. Genes 11(6):633. https://doi.org/10.3390/genes11060633
Buzas DM, Gresshoff PM (2007) Short- and long-distance control of root development by LjHAR1 during the juvenile stage of Lotus japonicus. J Plant Physiol 164(4):452–459. https://doi.org/10.1016/j.jplph.2006.03.006
Caetano-Anollés G, Gresshoff PM (1991) Alfalfa controls nodulation during the onset of Rhizobium-induced cortical cell division. Plant Physiol 95(2):366–373. https://doi.org/10.1104/pp.95.2.366
Carroll BJ, McNeil DL, Gresshoff PM (1985) Isolation and properties of soybean [Glycine max (L.) Merr.] mutants that nodulate in the presence of high nitrate concentrations. Proc Natl Acad Sci USA 82(12):4162–4166. https://doi.org/10.1073/pnas.82.12.4162
Chaulagain D, Frugoli J (2021) The regulation of nodule number in negumes is a balance of three signal transduction pathways. Int J Mol Sci 22(3):117. https://doi.org/10.3390/ijms22031117
Chen X, Yao Q, Gao X, Jiang C, Harberd NP, Fu X (2016) Shoot-to-root mobile transcription factor HY5 coordinates plant carbon and nitrogen acquisition. Curr Biol 26(5):640–646. https://doi.org/10.1016/j.cub.2015.12.066
Dong W, Wang Y, Takahashi H (2019) CLE-CLAVATA1 signaling pathway modulates lateral root development under sulfur deficiency. Plants 8(4):103. https://doi.org/10.3390/plants8040103
Ferguson BJ, Mens C, Hastwell AH, Zhang M, Su H, Jones CH, Chu X, Gresshoff PM (2019) Legume nodulation: the host controls the party. Plant Cell Environ 42(1):41–51. https://doi.org/10.1111/pce.13348
Forde BG (2014) Nitrogen signalling pathways shaping root system architecture: an update. Curr Opin Plant Biol 21:30–36. https://doi.org/10.1016/j.pbi.2014.06.004
Fredes I, Moreno S, Díaz FP, Gutiérrez RA (2019) Nitrate signaling and the control of Arabidopsis growth and development. Curr Opin Plant Biol 47:112–118. https://doi.org/10.1016/j.pbi.2018.10.004
Fujita H, Hayashi-Tsugane M, Kawaguchi M (2020) Spatial regulation of resource allocation in response to nutritional availability. J Theor Biol 486:110078. https://doi.org/10.1016/j.jtbi.2019.110078
Gautrat P, Laffont C, Frugier F (2020) Compact Root Architecture 2 promotes root competence for nodulation through the miR2111 systemic effector. Curr Biol 30(7):1339-1345.e3. https://doi.org/10.1016/j.cub.2020.01.084
Giehl RFH, von Wirén N (2014) Root nutrient foraging. Plant Physiol 166(2):509–517. https://doi.org/10.1104/pp.114.245225
Goh CH, Nicotra AB, Mathesius U (2019) Genes controlling legume nodule numbers affect phenotypic plasticity responses to nitrogen in the presence and absence of rhizobia. Plant Cell Environ 42(5):1747–1757. https://doi.org/10.1111/pce.13498
Gruber BD, Giehl RFH, Friedel S, von Wirén N (2013) Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol 163(1):161–179. https://doi.org/10.1104/pp.113.218453
Imin N, Patel N, Corcilius L, Payne RJ, Djordjevic MA (2018) CLE peptide tri-arabinosylation and peptide domain sequence composition are essential for SUNN-dependent autoregulation of nodulation in Medicago truncatula. New Phytol 218(1):73–80. https://doi.org/10.1111/nph.15019
Jung JKH, McCouch S (2013) Getting to the roots of it: Genetic and hormonal control of root architecture. Front Plant Sci 4:186. https://doi.org/10.3389/fpls.2013.00186
Kawaguchi M (2000) Lotus japonicus “Miyakojima” MG-20: an early-flowering accession suitable for indoor handling. J Plant Res 113:507–509. https://doi.org/10.1007/PL00013961
Kawaguchi M, Imaizumi-Anraku H, Koiwa H, Niwa S, Ikuta A, Syono K, Akao S (2002) Root, root hair, and symbiotic mutants of the model legume Lotus japonicus. Mol Plant-Microbe Interact 15(1):17–26. https://doi.org/10.1094/MPMI.2002.15.1.17
Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, Mounier E, Hoyerova K, Tillard P, Leon S, Ljung K, Zazimalova E, Benkova E, Nancry P, Gojon A (2010) Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell 18(9):927–937. https://doi.org/10.1016/j.devcel.2010.05.008
Krusell L, Madsen LH, Sato S, Aubert G, Genua A, Szczyglowski K, Duc G, Kaneko T, Tabata S, de Bruijn F, Pajuelo E, Sandal N, Stougaard J (2002) Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature 420(6914):422–426. https://doi.org/10.1038/nature01207
Laffont C, Ivanovici A, Gautrat P, Brault M, Djordjevic MA, Frugier F (2020) The NIN transcription factor coordinates CEP and CLE signaling peptides that regulate nodulation antagonistically. Nat Commun 11(1):3167. https://doi.org/10.1038/s41467-020-16968-1
Lebedeva M, Azarakhsh M, Yashenkova Y, Lutova L (2020) Nitrate-induced CLE peptide systemically inhibits nodulation in Medicago truncatula. Plants 9(11):1456. https://doi.org/10.3390/plants9111456
Lim CW, Lee YW, Lee SC, Hwang CH (2014) Nitrate inhibits soybean nodulation by regulating expression of CLE genes. Plant Sci 229:1–9. https://doi.org/10.1016/j.plantsci.2014.08.014
Magori S, Oka-Kira E, Shibata S, Umehara Y, Kouchi H, Hase Y, Tanaka A, Sato S, Tabata S, Kawaguchi M (2009) TOO MUCH LOVE, a root regulator associated with the long-distance control of nodulation in Lotus japonicus. Mol Plant-Microbe Interact 22(3):259–268. https://doi.org/10.1094/MPMI-22-3-0259
Mens C, Hastwell AH, Su H, Gresshoff PM, Mathesius U, Ferguson BJ (2021) Characterisation of Medicago truncatula CLE34 and CLE35 in nitrate and rhizobia regulation of nodulation. New Phytol 229(5):2525–2534. https://doi.org/10.1111/nph.17010
Moreau C, Gautrat P, Frugier F (2021) Nitrate-induced CLE35 signaling peptides inhibit nodulation through the SUNN receptor and miR2111 repression. Plant Physiol 185(3):1216–1228. https://doi.org/10.1093/plphys/kiaa094
Mortier V, Den Herder G, Whitford R, Van de Velde W, Rombauts S, D’Haeseleer K, Holsters M, Goormachtig S (2010) CLE peptides control Medicago truncatula nodulation locally and systemically. Plant Physiol 153(1):222–237. https://doi.org/10.1104/pp.110.153718
Nagae M, Parniske M, Kawaguchi M, Takeda N (2016) The thiamine biosynthesis gene THI1 promotes nodule growth and seed maturation. Plant Physiol 172(3):2033–2043. https://doi.org/10.1104/pp.16.01254
Nishida H, Handa Y, Tanaka S, Suzaki T, Kawaguchi M (2016) Expression of the CLE-RS3 gene suppresses root nodulation in Lotus japonicus. J Plant Res 129(5):909–919. https://doi.org/10.1007/s10265-016-0842-z
Nishida H, Suzaki T (2018) Nitrate-mediated control of root nodule symbiosis. Curr Opin Plant Biol 44:129–136. https://doi.org/10.1016/j.pbi.2018.04.006
Nishida H, Tanaka S, Handa Y, Ito M, Sakamoto Y, Matsunaga S, Betsuyaku S, Miura K, Soyano T, Kawaguchi M, Suzaki T (2018) A NIN-LIKE PROTEIN mediates nitrate-induced control of root nodule symbiosis in Lotus japonicus. Nat Commun 9(1):499. https://doi.org/10.1038/s41467-018-02831-x
Nishimura R, Hayashi M, Wu GJ, Kouchi H, Imaizumi-Anraku H, Murakami Y, Kawasaki S, Akao S, Ohmori M, Nagasawa M, Harada K, Kawaguchi M (2002) HAR1 mediates systemic regulation of symbiotic organ development. Nature 420(6914):426–429. https://doi.org/10.1038/nature01231
Nutman PS (1952) Host-factors influencing infection and nodule development in leguminous plants. Proc R Soc Lond B Biol Sci 139(895):176–185. https://doi.org/10.1098/rspb.1952.0003
O’Brien JA, Vega A, Bouguyon E, Krouk G, Gojon A, Coruzzi G, Gutiérrez RA (2016) Nitrate transport, sensing, and responses in plants. Mol Plant 9(6):837–856. https://doi.org/10.1016/j.molp.2016.05.004
Ohkubo Y, Tanaka M, Tabata R, Ogawa-Ohnishi M, Matsubayashi Y (2017) Shoot-to-root mobile polypeptides involved in systemic regulation of nitrogen acquisition. Nat Plants 3:17029. https://doi.org/10.1038/nplants.2017.29
Oka-Kira E, Kawaguchi M (2006) Long-distance signaling to control root nodule number. Curr Opin Plant Biol 9(5):496–502. https://doi.org/10.1016/j.pbi.2006.07.012
Okamoto S, Ohnishi E, Sato S, Takahashi H, Nakazono M, Tabata S, Kawaguchi M (2009) Nod factor/nitrate-induced CLE genes that drive HAR1-mediated systemic regulation of nodulation. Plant Cell Physiol 50(1):67–77. https://doi.org/10.1093/pcp/pcn194
Okamoto S, Shinohara H, Mori T, Matsubayashi Y, Kawaguchi M (2013) Root-derived CLE glycopeptides control nodulation by direct binding to HAR1 receptor kinase. Nat Commun 4:2191. https://doi.org/10.1038/ncomms3191
Okuma N, Soyano T, Suzaki T, Kawaguchi M (2020) MIR2111-5 locus and shoot-accumulated mature miR2111 systemically enhance nodulation depending on HAR1 in Lotus japonicus. Nat Commun 11(1):5192. https://doi.org/10.1038/s41467-020-19037-9
Osmont KS, Sibout R, Hardtke CS (2007) Hidden branches: developments in root system architecture. Annu Rev Plant Biol 58:93–113. https://doi.org/10.1146/annurev.arplant.58.032806.104006
Penmetsa RV, Frugoli JA, Smith LS, Long SR, Cook DR (2003) Dual genetic pathways controlling nodule number in Medicago truncatula. Plant Physiol 131(3):998–1008. https://doi.org/10.1104/pp.015677
Reid DE, Ferguson BJ, Gresshoff PM (2011) Inoculation- and nitrate-induced CLE peptides of soybean control NARK-dependent nodule formation. Mol Plant-Microbe Interact 24(5):606–618. https://doi.org/10.1094/MPMI-09-10-0207
Roy S, Liu W, Nandety RS, Crook A, Mysore KS, Pislariu CI, Frugoli J, Dickstein R, Udvardi MK (2020) Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. Plant Cell 32(1):15–41. https://doi.org/10.1105/tpc.19.00279
Ruffel S, Krouk G, Ristova D, Shasha D, Birnbaum KD, Coruzzi GM (2011) Nitrogen economics of root foraging: transitive closure of the nitrate-cytokinin relay and distinct systemic signaling for N supply vs. demand. Proc Natl Acad Sci USA 108(45):18524–18529. https://doi.org/10.1073/pnas.1108684108
Saito A, Tanabata S, Tanabata T, Tanabata T, Tajima S, Ueno M, Ishikawa S, Ohtake N, Sueyoshi K, Ohyama T (2014) Effect of nitrate on nodule and root growth of soybean (Glycine max (L.) Merr.). Int J Mol Sci 15(3):4464–4480. https://doi.org/10.3390/ijms15034464
Schnabel E, Journet E-P, de Carvalho-Niebel F, Duc G, Frugoli J (2005) The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Mol Biol 58(6):809–822. https://doi.org/10.1007/s11103-005-8102-y
Searle IR, Men AE, Laniya TS, Buzas DM, Iturbe-Ormaetxe I, Carroll BJ, Gresshoff PM (2003) Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science 299(5603):109–112. https://doi.org/10.1126/science.1077937
Shahzad Z, Amtmann A (2017) Food for thought: how nutrients regulate root system architecture. Curr Opin Plant Biol 39:80–87. https://doi.org/10.1016/j.pbi.2017.06.008
Soyano T, Hirakawa H, Sato S, Hayashi M, Kawaguchi M (2014) Nodule inception creates a long-distance negative feedback loop involved in homeostatic regulation of nodule organ production. Proc Natl Acad Sci USA 111(40):14607–14612. https://doi.org/10.1073/pnas.1412716111
Streeter J, Wong PP (1988) Inhibition of legume nodule formation and N2 fixation by nitrate. Crit Rev Plant Sci 7:1–23. https://doi.org/10.1080/07352688809382257
Sun CH, Yu JQ, Hu DG (2017) Nitrate: a crucial signal during lateral roots development. Front Plant Sci 8:485. https://doi.org/10.3389/fpls.2017.00485
Suzaki T, Yoro E, Kawaguchi M (2015) Leguminous plants: inventors of root nodules to accommodate symbiotic bacteria. Int Rev Cell Mol Biol 316:111–158. https://doi.org/10.1016/bs.ircmb.2015.01.004
Tabata R, Sumida K, Yoshii T, Ohyama K, Shinohara H, Matsubayashi Y (2014) Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science 346(6207):343–346. https://doi.org/10.1126/science.1257800
Takahara M, Magori S, Soyano T, Okamoto S, Yoshida C, Yano K, Sato S, Tabata S, Yamaguchi K, Shigenobu S, Takeda N, Suzaki T, Kawaguchi M (2013) TOO MUCH LOVE, a novel Kelch repeat-containing F-box protein, functions in the long-distance regulation of the legume-Rhizobium symbiosis. Plant Cell Physiol 54(4):433–447. https://doi.org/10.1093/pcp/pct022
Tsikou D, Yan Z, Holt DB, Abel NB, Reid DE, Madsen LH, Bhasin H, Sexauer M, Stougaard J, Markmann K (2018) Systemic control of legume susceptibility to rhizobial infection by a mobile microRNA. Science 362(6411):233–236. https://doi.org/10.1126/science.aat6907
Wang C, Reid JB, Foo E (2020) The role of CLV1, CLV2 and HPAT homologues in the nitrogen-regulation of root development. Physiol Plant 170(4):607–621. https://doi.org/10.1111/ppl.13200
Wopereis J, Pajuelo E, Dazzo FB, Jiang Q, Gresshoff PM, de Bruijin FJ, Stougaard J, Szczyglowski K (2000) Short root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype. Plant J 23(1):97–114. https://doi.org/10.1046/j.1365-313x.2000.00799.x
Yoro E, Nishida H, Ogawa-Ohnishi M, Yoshida C, Suzaki T, Matsubayashi Y, Kawaguchi M (2019) PLENTY, a hydroxyproline O-arabinosyltransferase, negatively regulates root nodule symbiosis in Lotus japonicus. J Exp Bot 70(2):507–517. https://doi.org/10.1093/jxb/ery364
Zhang H, Jennings A, Barlow PW, Forde BG (1999) Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci USA 96(11):6529–6534. https://doi.org/10.1073/pnas.96.11.6529
Zhang M, Su H, Gresshoff PM, Ferguson BJ (2021) Shoot-derived miR2111 controls legume root and nodule development. Plant Cell Environ 44(5):1627–1641. https://doi.org/10.1111/pce.13992
Acknowledgements
We thank Dr. Nao Okuma for helpful technical advice on the grafting experiment and qRT-PCR analysis. This work was supported by JSPS KAKENHI (17H03702, 20H03283) to MK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Fig. S1
Effects of nitrate supply on root morphology in WT, har1 and tml plants. a Primary root length (PRL). b Total lateral root (LR) number per plant. Numbers of first (c) and second (d) LRs. e Total LR length (LRL) per plant. Plants were grown in 0.03, 0.3, 3, or 30 mM nitrate for 12 days (n = 10 plants). Boxplots show the median (thick line), quartiles (box), minimum and maximum ranges (lines), and outliers (circles). Different letters represent significant differences across nitrate concentrations and plant genotypes (one-way ANOVA, post hoc Tukey’s test, P < 0.05). Fig. S2 Effects of nitrate supply on the expression of AON-related genes. Gene expression levels of CLE-RS2 (a), TML (b), and HAR1 (c) in shoots and roots of WT plants grown in 0.03, 0.3, 3, or 30 mM nitrate for 12 days. ubiquitin (UBQ) was used as a reference gene. Different letters represent significant differences across plant genotypes (one-way ANOVA, post hoc Tukey’s test, P < 0.05). Error bars indicate ± SE from three biological replicates (PPTX 118 KB)
Rights and permissions
About this article
Cite this article
Hayashi-Tsugane, M., Kawaguchi, M. Lotus japonicus HAR1 regulates root morphology locally and systemically under a moderate nitrate condition in the absence of rhizobia. Planta 255, 95 (2022). https://doi.org/10.1007/s00425-022-03873-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-022-03873-8