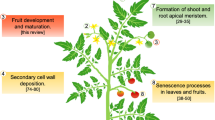
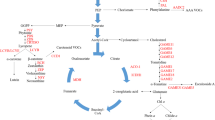
Abstract
Long-term goals to impact or modify fruit quality and yield have been the target of researchers for many years. Different approaches such as traditional breeding, mutation breeding, and transgenic approaches have revealed a regulatory network where several hormones concur in a complex way to regulate fruit set and development, and these networks are shared in some way among species with different kinds of fruits. Understanding the molecular and biochemical networks of fruit set and development could be very useful for breeders to meet the current and future challenges of agricultural problems.


Similar content being viewed by others
References
Alabadi D, Blazquez MA, Carbonell J, Ferrandiz C, Perez-Amador MA (2009) Instructive roles for hormones in plant development. Int J Dev Biol 53:1597–1608
Aloni R, Aloni E, Langhans M, Ullrich CI (2006) Role of auxin in regulating Arabidopsis flower development. Planta 223:315–328
Alvarez-Buylla ER, Benitez M, Corvera-Poirao A, Chaos Cador A, de Folter S, de Gamboa de Buen A, Garay-Arroyo A, Garcia-Ponce B, Jaimes-Miranda F, Perez-Ruiz RV, Pineyro-Nelson A, Sanchez-Corrales YE (2010) Flower development. Arabidopsis Book 8:e0127. doi:10.1199/tab.0127
Ampomah-Dwamena C, Morris BA, Sutherland P, Veit B, Yao J-L (2002) Down-regulation of TM29, a TomatoSEPALLATA homolog, causes parthenocarpic fruit development and floral reversion. Plant Physiol 130:605–617
Baker SC, Robinson-Beers K, Villanueva JM, Gaiser JC, Gasser CS (1997) Interactions among genes regulating ovule development in Arabidopsis thaliana. Genetics 145:1109–1124
Balanza V, Navarrete M, Trigueros M, Ferrandiz C (2006) Patterning the female side of Arabidopsis: the importance of hormones. J Exp Bot 57:3457–3469
Balbi V, Lomax TL (2003) Regulation of early tomato fruit development by the diageotropica gene. Plant Physiol 131:186–197
Bartrina I, Otto E, Strnad M, Werner T, Schmülling T (2011) Cytokinin regulates the activity of reproductive Meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell 23:69–80
Battaglia R, Colombo M, Kater MM (2009) The ins and outs of ovule development. Annual plant reviews volume 38: fruit development and seed dispersal. Wiley-Blackwell, New York, pp 70–106
Bemer M, Karlova R, Ballester AR, Tikunov YM, Bovy AG, Wolters-Arts M, Rossetto Pde B, Angenent GC, de Maagd RA (2012) The tomato FRUITFULL homologs TDR4/FUL1 and MBP7/FUL2 regulate ethylene-independent aspects of fruit ripening. Plant Cell 24:4437–4451
Bencivenga S, Simonini S, Benková E, Colombo L (2012) The transcription factors BEL1 and SPL are required for cytokinin and auxin signaling during ovule development in Arabidopsis. Plant Cell 24:2886–2897
Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602
Berger F, Hamamura Y, Ingouff M, Higashiyama T (2008) Double fertilization—caught in the act. Trends Plant Sci 13:437–443
Carbonell-Bejerano P, Urbez C, Carbonell J, Granell A, Perez-Amador MA (2010) A fertilization-independent developmental program triggers partial fruit development and senescence processes in pistils of Arabidopsis. Plant Physiol 154:163–172
Carbonell-Bejerano P, Urbez C, Granell A, Carbonell J, Perez-Amador M (2011) Ethylene is involved in pistil fate by modulating the onset of ovule senescence and the GA-mediated fruit set in Arabidopsis. BMC Plant Biol 11:84
Carmi N, Salts Y, Dedicova B, Shabtai S, Barg R (2003) Induction of parthenocarpy in tomato via specific expression of the rolB gene in the ovary. Planta 217:726–735
Carrera E, Ruiz-Rivero O, Peres LEP, Atares A, Garcia-Martinez JL (2012) Characterization of the procera tomato mutant shows novel functions of the SlDELLA protein in the control of flower morphology, cell division and expansion, and the auxin-signaling pathway during fruit-set and development. Plant Physiol 160:1581–1596
de Folter S, Busscher J, Colombo L, Losa A, Angenent G (2004) Transcript profiling of transcription factor genes during silique development in Arabidopsis. Plant Mol Biol 56:351–366
de Jong M, Mariani C, Vriezen WH (2009a) The role of auxin and gibberellin in tomato fruit set. J Exp Bot 60:1523–1532
de Jong M, Wolters-Arts M, Feron R, Mariani C, Vriezen WH (2009b) The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant J 57:160–170
de Jong M, Wolters-Arts M, Garcia-Martinez JL, Mariani C, Vriezen WH (2011) The Solanum lycopersicum AUXIN RESPONSE FACTOR 7 (SlARF7) mediates cross-talk between auxin and gibberellin signalling during tomato fruit set and development. J Exp Bot 62:617–626
Ding J, Chen B, Xia X, Mao W, Shi K, Zhou Y, Yu J (2013) Cytokinin-induced parthenocarpic fruit development in tomato is partly dependent on enhanced gibberellin and auxin biosynthesis. PLoS One 8:e70080
Donzella G, Spena A, Rotino GL (2000) Transgenic parthenocarpic eggplants: superior germplasm for increased winter production. Mol Breed 6:79–86
Dorcey E, Urbez C, Blázquez MA, Carbonell J, Perez-Amador MA (2009) Fertilization-dependent auxin response in ovules triggers fruit development through the modulation of gibberellin metabolism in Arabidopsis. Plant J 58:318–332
Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker W, Gerentes D, Perez P, Smyth DR (1996) AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell 8:155–168
Fischer R, Budde I, Hain R (1997) Stilbene synthase gene expression causes changes in flower colour and male sterility in tobacco. Plant J 11:489–498
Fos M, Nuez F, García-Martínez JL (2000) The Gene pat-2, which induces natural parthenocarpy, alters the Gibberellin content in unpollinated tomato ovaries. Plant Physiol 122:471–480
Fos M, Proano K, Nuez F, Garcia-Martinez JL (2001) Role of gibberellins in parthenocarpic fruit development induced by the genetic system pat-3/pat-4 in tomato. Physiol Plant 111:545–550
Fuentes S, Ljung K, Sorefan K, Alvey E, Harberd NP, Ostergaard L (2012) Fruit growth in Arabidopsis occurs via DELLA-dependent and DELLA-independent gibberellin responses. Plant Cell 24:3982–3996
Fuentes S, Vivian-Smith A (2009) Fertilisation and fruit initiation. Annual plant reviews volume 38: fruit development and seed dispersal. Wiley-Blackwell, New York, pp 107–171
Fujisawa M, Ito Y (2013) The regulatory mechanism of fruit ripening revealed by analyses of direct targets of the tomato MADS-box transcription factor RIPENING INHIBITOR. Plant Signal Behav 8(6): e24357 [Epub ahead of print]
Fujisawa M, Nakano T, Shima Y, Ito Y (2013) A large-scale identification of direct targets of the tomato MADS box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening. Plant Cell 25:371–386
Fujisawa M, Shima Y, Higuchi N, Nakano T, Koyama Y, Kasumi T, Ito Y (2012) Direct targets of the tomato-ripening regulator RIN identified by transcriptome and chromatin immunoprecipitation analyses. Planta 235:1107–1122
García-Hurtado N, Carrera E, Ruiz-Rivero O, López-Gresa MP, Hedden P, Gong F, García-Martínez JL (2012) The characterization of transgenic tomato overexpressing gibberellin 20-oxidase reveals induction of parthenocarpic fruit growth, higher yield, and alteration of the gibberellin biosynthetic pathway. J Exp Bot 63:5803–5813
Garcia-Martinez JL, Lopez-Diaz I, Sanchez-Beltran MJ, Phillips AL, Ward DA, Gaskin P, Hedden P (1997) Isolation and transcript analysis of gibberellin 20-oxidase genes in pea and bean in relation to fruit development. Plant Mol Biol 33:1073–1084
Gillaspy G, Ben-David H, Gruissem W (1993) Fruits: a developmental perspective. Plant Cell 5(10):1439–1451
Giovannoni JJ (2004) Genetic regulation of fruit development and ripening. Plant Cell 16:S170–S180
Giovannoni JJ (2006) Breeding new life into plant metabolism. Nat Biotech 24:418–419
Goetz M, Hooper LC, Johnson SD, Rodrigues JCM, Vivian-Smith A, Koltunow AM (2007) Expression of aberrant forms of AUXIN RESPONSE FACTOR8 stimulates parthenocarpy in Arabidopsis and tomato. Plant Physiol 145:351–366
Goetz M, Vivian-Smith AD, Johnson S, Koltunow AM (2006) Auxin Response Factor 8 is a negative regulator of fruit initiation in Arabidopsis. Plant Cell 18:1873–1886
Gorguet B, van Heusden AW, Lindhout P (2005) Parthenocarpic fruit development in tomato. Plant Biol 7:131–139
Gustafson F (1942) Parthenocarpy: natural and artificial. Bot Rev 8:599–654
Hu J, Mitchum MG, Barnaby N, Ayele BT, Ogawa M, Nam E, Lai W-C, Hanada A, Alonso JM, Ecker JR, Swain SM, Yamaguchi S, Kamiya Y, Sun T-p (2008) Potential sites of bioactive Gibberellin production during reproductive growth in Arabidopsis. Plant Cell 20:320–336
Ingrosso I, Bonsegna S, De Domenico S, Laddomada B, Blando F, Santino A, Giovinazzo G (2011) Over-expression of a grape stilbene synthase gene in tomato induces parthenocarpy and causes abnormal pollen development. Plant Physiol Biochem 49:1092–1099
Itkin M, Seybold H, Breitel D, Rogachev I, Meir S, Aharoni A (2009) TOMATO AGAMOUS-LIKE 1 is a component of the fruit ripening regulatory network. Plant J 60:1081–1095
Ito T, Meyerowitz EM (2000) Overexpression of a gene encoding a cytochrome P450, CYP78A9, induces large and seedless fruit in Arabidopsis. The Plant Cell 12:1541–1550
Karlova R, Rosin FM, Busscher-Lange J, Parapunova V, Do PT, Fernie AR, Fraser PD, Baxter C, Angenent GC, de Maagd RA (2011) Transcriptome and metabolite profiling show that APETALA2a is a major regulator of tomato fruit ripening. Plant Cell 23:923–941
Lang JD, Ray S, Ray A (1994) sin1, a mutation affecting female fertility in Arabidopsis, interacts with mod1, its recessive modifier. Genetics 137:1101–1110
León-Kloosterziel K, Keijzer C, Koornneef M (1994) A seed shape mutant of Arabidopsis that is affected in integument development. Plant Cell 6:385–392
Lora J, Hormaza JI, Herrero M, Gasser CS (2011) Seedless fruits and the disruption of a conserved genetic pathway in angiosperm ovule development. Proc Natl Acad Sci USA 108:5461–5465
Mahajan M, Ahuja PS, Yadav SK (2011) Post-transcriptional silencing of flavonol synthase mRNA in tobacco leads to fruits with arrested seed set. PLoS ONE 6:e28315
Mariotti L, Picciarelli P, Lombardi L, Ceccarelli N (2011) Fruit-set and early fruit growth in tomato are associated with increases in indoleacetic acid, cytokinin, and bioactive Gibberellin contents. J Plant Growth Regul 30:405–415
Marsch-Martinez N, Greco R, Van Arkel G, Herrera-Estrella L, Pereira A (2002) Activation tagging using the En-I Maize transposon system in Arabidopsis. Plant Physiol 129:1544–1556
Marsch-Martinez N, Ramos-Cruz D, Irepan Reyes-Olalde J, Lozano-Sotomayor P, Zuñiga-Mayo VM, de Folter S (2012) The role of cytokinin during Arabidopsis gynoecia and fruit morphogenesis and patterning. Plant J 72:222–234
Martí C, Orzáez D, Ellul P, Moreno V, Carbonell J, Granell A (2007) Silencing of DELLA induces facultative parthenocarpy in tomato fruits. Plant J 52:865–876
Matsuo S, Kikuchi K, Fukuda M, Honda I, Imanishi S (2012) Roles and regulation of cytokinins in tomato fruit development. J Exp Bot 63:5569–5579
Mazzucato A, Olimpieri I, Siligato F, Picarella ME, Soressi GP (2008) Characterization of genes controlling stamen identity and development in a parthenocarpic tomato mutant indicates a role for the DEFICIENS ortholog in the control of fruit set. Physiol Plant 132:526–537
McAtee P, Karim S, Schaffer RJ, David K (2013) A dynamic interplay between phytohormones is required for fruit development, maturation and ripening. Front Plant Sci 4
Medina M, Roque E, Pineda B, Cañas L, Rodriguez-Concepción M, Beltrán JP, Gómez-Mena C (2013) Early anther ablation triggers parthenocarpic fruit development in tomato. Plant Biotechnol J 11:770–779
Mezzetti B, Landi L, Pandolfini T, Spena A (2004) The defH9-iaaM auxin-synthesizing gene increases plant fecundity and fruit production in strawberry and raspberry. BMC Biotechnol 4:4
Mignolli F, Mariotti L, Lombardi L, Vidoz ML, Ceccarelli N, Picciarelli P (2012) Tomato fruit development in the auxin-resistant dgt mutant is induced by pollination but not by auxin treatment. J Plant Physiol 169:1165–1172
Mizzotti C, Mendes MA, Caporali E, Schnittger A, Kater MM, Battaglia R, Colombo L (2011) The MADS box genes SEEDSTICK and ARABIDOPSIS Bsister play a maternal role in fertilization and seed development. Plant J 70:409–420
Modrusan Z, Reiser L, Feldmann KA, Fischer RL, Haughn GW (1994) Homeotic transformation of ovules into carpel-like structures in Arabidopsis. Plant Cell 6:333–349
Molesini B, Pandolfini T, Rotino GL, Dani V, Spena A (2009) Aucsia gene silencing causes parthenocarpic fruit development in tomato. Plant Physiol 149:534–548
Mounet F, Moing A, Kowalczyk M, Rohrmann J, Petit J, Garcia V, Maucourt M, Yano K, Deborde C, Aoki K, Bergès H, Granell A, Fernie AR, Bellini C, Rothan C, Lemaire-Chamley M (2012) Down-regulation of a single auxin efflux transport protein in tomato induces precocious fruit development. J Exp Bot 63:4901–4917
Nishitani C, Yamaguchi-Nakamura A, Hosaka F, Terakami S, Shimizu T, Yano K, Itai A, Saito T, Yamamoto T (2012) Parthenocarpic genetic resources and gene expression related to parthenocarpy among four species in pear (Pyrus spp.). Scientia Horticulturae 136:101–109
Oh K, Ivanchenko MG, White TJ, Lomax TL (2006) The diageotropica gene of tomato encodes a cyclophilin: a novel player in auxin signaling. Planta 224:133–144
Ozga J, Reinecke D (2003) Hormonal interactions in fruit development. J Plant Growth Regul 22:73–81
Pandolfini T (2009) Seedless fruit production by hormonal regulation of fruit set. Nutrients 1:168–177
Pandolfini T, Molesini B, Spena A (2009) Parthenocarpy in crop plants. Annual plant reviews volume 38: fruit development and seed dispersal. Wiley-Blackwell, New York, pp 326–345
Pandolfini T, Rotino G, Camerini S, Defez R, Spena A (2002) Optimisation of transgene action at the post-transcriptional level: high quality parthenocarpic fruits in industrial tomatoes. BMC Biotechnol 2:1
Pascual L, Blanca JM, Canizares J, Nuez F (2009) Transcriptomic analysis of tomato carpel development reveals alterations in ethylene and gibberellin synthesis during pat3/pat4 parthenocarpic fruit set. BMC Plant Biol 9:1471–2229
Payne T, Johnson SD, Koltunow AM (2004) KNUCKLES (KNU) encodes a C2H2 zinc-finger protein that regulates development of basal pattern elements of the Arabidopsis gynoecium. Development 131:3737–3749
Pinyopich A, Ditta GS, Savidge B, Liljegren SJ, Baumann E, Wisman E, Yanofsky MF (2003) Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature 424:85–88
Qin G, Wang Y, Cao B, Wang W, Tian S (2012) Unraveling the regulatory network of the MADS box transcription factor RIN in fruit ripening. Plant J 70:243–255
Ray A, Robinson-Beers K, Ray S, Baker SC, Lang JD, Preuss D, Milligan SB, Gasser CS (1994) Arabidopsis floral homeotic gene BELL (BEL1) controls ovule development through negative regulation of AGAMOUS gene (AG). Proc Natl Acad Sci USA 91:5761–5765
Ren Z, Li Z, Miao Q, Yang Y, Deng W, Hao Y (2011) The auxin receptor homologue in Solanum lycopersicum stimulates tomato fruit set and leaf morphogenesis. J Exp Bot 62:2815–2826
Reyes-Olalde JI, Zuniga-Mayo VM, Chavez Montes RA, Marsch-Martinez N, de Folter S (2013) Inside the gynoecium: at the carpel margin. Trends Plant Sci 2013:00151–00159
Rieu I, Eriksson S, Powers SJ, Gong F, Griffiths J, Woolley L, Benlloch R, Nilsson O, Thomas SG, Hedden P, Phillips AL (2008a) Genetic analysis reveals that C19-GA 2-oxidation is a major Gibberellin inactivation pathway in Arabidopsis. Plant Cell 20:2420–2436
Rieu I, Ruiz-Rivero O, Fernandez-Garcia N, Griffiths J, Powers SJ, Gong F, Linhartova T, Eriksson S, Nilsson O, Thomas SG, Phillips AL, Hedden P (2008b) The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant J 53:488–504
Roeder AHK, Yanofsky MF (2006) Fruit development in Arabidopsis. Arabidopsis Book 4:e0075. doi:10.1199/tab.0075
Rotino GL, Acciarri N, Sabatini E, Mennella G, Lo Scalzo R, Maestrelli A, Molesini B, Pandolfini T, Scalzo J, Mezzetti B, Spena A (2005) Open field trial of genetically modified parthenocarpic tomato: seedlessness and fruit quality. BMC Biotechnol 5:32
Rotino GL, Perri E, Zottini M, Sommer H, Spena A (1997) Genetic engineering of parthenocarpic plants. Nat Biotechnol 15:1398–1401
Sauer M, Kleine-Vehn J (2011) AUXIN BINDING PROTEIN1: the outsider. Plant Cell 23:2033–2043
Scherer GF (2011) AUXIN-BINDING-PROTEIN1, the second auxin receptor: what is the significance of a two-receptor concept in plant signal transduction? J Exp Bot 62:3339–3357
Schijlen EGWM, de Vos CHR, Martens S, Jonker HH, Rosin FM, Molthoff JW, Tikunov YM, Angenent GC, van Tunen AJ, Bovy AG (2007) RNA interference silencing of chalcone synthase, the first step in the flavonoid biosynthesis pathway, leads to parthenocarpic tomato fruits. Plant Physiol 144:1520–1530
Serrani JC, Carrera E, Ruiz-Rivero O, Gallego-Giraldo L, Peres LEP, Garcia-Martinez JL (2010) Inhibition of auxin transport from the ovary or from the apical shoot induces parthenocarpic fruit-set in tomato mediated by gibberellins. Plant Physiol 153:851–862
Serrani JC, Sanjuán R, Ruiz-Rivero O, Fos M, García-Martínez JL (2007) Gibberellin regulation of fruit set and growth in tomato. Plant Physiol 145:246–257
Seymour GB, Østergaard L, Chapman NH, Knapp S, Martin C (2013) Fruit development and ripening. Annu Rev Plant Biol 64:219–241
Sotelo-Silveira M, Cucinotta M, Colombo L, Marsch-Martinez N, de Folter S (2013b) Toward understanding the role of CYP78A9 during Arabidopsis reproduction. Plant Signal Behav 8(8): e25160 [Epub ahead of print]
Sotelo-Silveira M, Cucinotta M, Chauvin AL, Chavez Montes RA, Colombo L, Marsch-Martinez N, de Folter S (2013a) Cytochrome P450 CYP78A9 is involved in Arabidopsis reproductive development. Plant Physiol 162:779–799
Sun T-P (2008) Gibberellin Metabolism, Perception and Signaling Pathways in Arabidopsis. Arabidopsis Book 6:e0103. doi:10.1199/tab.0103
Sundberg E, Ferrándiz C (2009) Gynoecium patterning in Arabidopsis: a basic plan behind a complex structure. Annual plant reviews volume 38: fruit development and seed dispersal. Wiley-Blackwell, New York, pp 35–69
Talon M, Zacarias L, Primo-Millo E (1992) Gibberellins and parthenocarpic ability in developing ovaries of seedless mandarins. Plant Physiol 99:1575–1581
Tiwari A, Offringa R, Heuvelink E (2012) Auxin-induced fruit set in Capsicum annuum L. requires downstream gibberellin biosynthesis. J Plant Growth Regul 31:570–578
Tiwari A, Vivian-Smith A, Voorrips R, Habets M, Xue L, Offringa R, Heuvelink E (2011) Parthenocarpic potential in Capsicum annuum L. is enhanced by carpelloid structures and controlled by a single recessive gene. BMC Plant Biol 11:143
Villanueva JM, Broadhvest J, Hauser BA, Meister RJ, Schneitz K, Gasser CS (1999) INNER NO OUTER regulates abaxial- adaxial patterning in Arabidopsis ovules. Genes Dev 13:3160–3169
Vivian-Smith A, Koltunow AM (1999) Genetic analysis of growth-regulator-induced parthenocarpy in Arabidopsis. Plant Physiol 121:437–452
Vivian-Smith A, Luo M, Chaudhury A, Koltunow A (2001) Fruit development is actively restricted in the absence of fertilization in Arabidopsis. Development 128:2321–2331
Vrebalov J, Pan IL, Arroyo AJM, McQuinn R, Chung M, Poole M, Rose J, Seymour G, Grandillo S, Giovannoni J, Irish VF (2009) Fleshy fruit expansion and ripening are regulated by the tomato SHATTERPROOF gene TAGL1. Plant Cell 21:3041–3062
Vriezen WH, Feron R, Maretto F, Keijman J, Mariani C (2008) Changes in tomato ovary transcriptome demonstrate complex hormonal regulation of fruit set. New Phytol 177:60–76
Wang H, Jones B, Li Z, Frasse P, Delalande C, Regad F, Chaabouni S, Latché A, Pech J-C, Bouzayen M (2005) The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 17:2676–2692
Wang L, Xin M, Qin Z, Liu H (2011) Functional analysis of an iaaM gene in parthenocarpic fruit development in transgenic Physalis pubescens L. plants. Plant Cell Tissue Organ Culture 107:333–340
Yao J-L, Dong Y-H, Morris BAM (2001) Parthenocarpic apple fruit production conferred by transposon insertion mutations in a MADS-box transcription factor. Proc Natl Acad Sci USA 98:1306–1311
Yin Z, Malinowski R, Ziółkowska A, Sommer H, Plcader W, Malepszy S (2006) The DefH9-IaaM-containing construct efficiently induces parthenocarpy in cucumber. Cell Mol Biol Lett 11:279–290
Acknowledgments
We would like to thank the anonymous reviewers for their very helpful comments on this review. We thank the Mexican National Council of Science and Technology (CONACyT) for a PhD fellowship to MSS (229496). This work in the de Folter laboratory is financed by the CONACyT Grant 177739.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sotelo-Silveira, M., Marsch-Martínez, N. & de Folter, S. Unraveling the signal scenario of fruit set. Planta 239, 1147–1158 (2014). https://doi.org/10.1007/s00425-014-2057-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-014-2057-7