Abstract
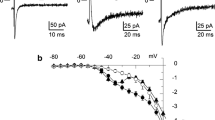
Effects of synthesized calciseptine (CaS), found naturally in the venom of the black mamba, on voltage-dependent Ca2+ channels in smooth muscle cells of the guinea-pig portal vein were investigated. In the whole-cell voltage-clamp configuration, extracellular application of CaS (≥ 10 nM) inhibited the inward current in a concentration- and voltage-dependent manner at a holding potential of −90 mV. The Ca2+ current recorded at a high holding potential (−50 mV) was approximately 8 times more sensitive to CaS than that at a more negative holding potential (−90 mV). CaS (50 nM) shifted to the left the steady-state inactivation curve obtained by using single 8-s conditioning pulses of various amplitudes. When CaS (≥ 200 nM) was present in the pipette, the Ca2+ current remained for the duration of the experiments (more than 60 min) in the whole-cell configuration. Two different Ca2+ channel conductances are present in this tissue (25-pS and 12-pS channels). Both channels are blocked by dihydropyridine (DHP) derivatives, but have different sensitivities. In the cell-attached condition, CaS hardly changed the activity of either unitary Ca2+ channel current. To prevent the “run down” of the Ca2+ channels in cell-free conditions, we added cardiac cytosol, a supernatant from homogenized cardiac cells and an endogenous Ca2+ channel activating factor, in the pipette. The unitary Ca2+ channel currents were then recorded using the outside-out membrane patch configuration. Application of CaS (1 μM) in the bath completely blocked the open events of the 25-pS Ca2+ channel. CaS (10 nM) in the bath reduced the mean open time and channel availability, resulting in a decrease in the open probability of the 25-pS channel currents without affecting the amplitude of the single-channel conductance. CaS also reduced the open probability (though less potently) and channel availability of the 12-pS Ca2+ channel without a change in its amplitude. From these results, we conclude that CaS has inhibitory effects on the voltage-dependent Ca2+ current that are similar to those of DHP derivatives and that it acts from the outside of the membrane.
Similar content being viewed by others
References
Bean BP, Cohen CJ, Tsien RW (1983) Lidocaine block of cardiac sodium channels. J Gen Physiol 81:613–642
De Weille JR, Schweitz H, Maes P, Tartar A, Lazdunski M (1991) Calciseptine, a peptide isolated from black mamba venom, is a specific blocker of the L-type calcium channel. Proc Natl Acad Sci USA 88:2437–2440
Fish RD, Sperti G, Colucci WS, Clapham DE (1988) Phorbol ester increases the dihydropyridine-sensitive calcium conductance in a vascular smooth muscle cell line. Circ Res 62: 1049–1054
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391:85–100
Hess P, Lansman JB, Tsien RW (1984) Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature 311:538–544
Inoue Y, Xiong Z, Kitamura K, Kuriyama H (1989) Modulation produced by nifedipine of the unitary Ba current of dispersed smooth muscle cells of the rabbit ileum. Pflügers Arch 414:534–542
Inoue Y, Oike M, Nakao K, Kitamura K, Kuriyama H (1990) Endothelin augments unitary calcium channel currents on the smooth muscle cell membrane of guinea-pig portal vein. J Physiol (Lond) 423:171–191
Kameyama M, Kameyama A, Nakayama T, Kaibara M (1988) Tissue extract recovers cardiac calcium channels from “rundown”. Pflügers Arch 412:328–330
Kass RS, Arena JP, Chin S (1989) Cellular electrophysiology of amlodipine: probing the cardiac L-type calcium channel. Am J Cardiol 64:35 1–42 I
Kawashima Y, Ochi R (1988) Voltage-dependent decrease in the availability of single calcium channels by nitrendipine in guinea-pig ventricular cells. J Physiol (Lond) 402:219–235
Kuroda H, Chen Y-N, Watanabe TX, Kimura T, Sakakibara S (1992) Solution synthesis of calciseptine, an L-type specific calcium channel blocker. Peptide Res 5:265–268
Nakayama H, Taki M, Striessnig J, Glossmann H, Catterall WM, Kanaoka Y (1991) Identification of 1,4-dihydropyridine binding regions within the α1 subunit of skeletal muscle Ca2+ channels by photoaffmity labeling with diazepine. Proc Natl Acad Sci USA 88:9203–9207
Ohya Y, Terada K, Kitamura K, Kuriyama H (1987) D600 blocks the Ca2+ channel from the outer surface of smooth muscle cell membrane of the rabbit intestine and portal vein. Pflügers Arch 408:80–82
Regulla S, Schneider T, Nastainczyk W, Meyer HE, Hofmann F (1991) Identification of the site of interaction of the dihydropyridine channel blockers nitrendipine and azidopine with the calcium-channel al subunit. EMBO J 10:45–49
Sanguinetti MC, Kass RS (1984) Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res 55: 336–348
Tang S, Yatani A, Bahinski A, Mori Y, Schwartz A (1993) Molecular localization of regions in the L-type calcium channel critical for dihydropyridine action. Neuron 11:1013–1021
Terada K, Kitamura K, Kuriyama H (1987) Blocking actions of Ca2+ antagonists on the Ca2+ channels in the smooth muscle cell membrane of rabbit small intestine. Pflügers Arch 408:552–557
Uehara A, Hume JR (1985) Interactions of organic calcium channel antagonists with calcium channels in single frog atrial cells. J Gen Physiol 85:621–647
Watanabe TX, Itahara Y, Kuroda H, Chen YN, Kimura T, Sakakibara S (1995) Smooth muscle relaxing and hypotensive activities of synthetic calciseptine and the homologous snake venom peptide FS2. Jpn J Pharmacol 68:305–313
Yasuda O, Morimoto S, Chen Y, Jiang B, Kimura T, Sakakibara S, Koh E, Fukuo K, Kitano S, Ogihara T (1993) Calciseptine binding to a 1,4-dihydropyridine recognition site of the Ltype calcium channel of rat synaptosomal membranes. Biochem Biophys Res Commun 194:587–594
Zhang F, Ram JL, Standley PR, Sowers JR (1994) 17β-Estradiol attenuates voltage-dependent Ca2+ currents in A7r5 vascular smooth muscle cell line. Am J Physiol 266:C975-C980
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Teramoto, N., Ogata, R., Kuriyama, H. et al. Effects of calciseptine on unitary barium channel currents in guinea-pig portal vein. Pflügers Arch — Eur J Physiol 432, 462–470 (1996). https://doi.org/10.1007/s004240050158
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s004240050158