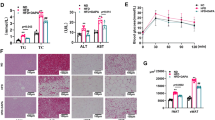
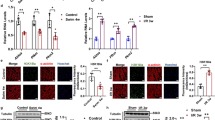
Abstract
The activation of beige fat and muscle tissues is an interesting and encouraging target for therapeutic intervention in obesity owing to their remarkable lipolytic activity and energy-consuming futile cycles. This study examined the effect of dopamine receptor D4 (DRD4) on lipid metabolisms as well as UCP1- and ATP-dependent thermogenesis in Drd4-silenced 3T3-L1 adipocytes and C2C12 muscle cells. Silencing of Drd4, followed by quantitative real-time PCR, immunoblot analysis, immunofluorescence, and staining methods, were applied to evaluate the effects of DRD4 on diverse target genes and proteins of both cells. The findings showed that DRD4 was expressed in the adipose and muscle tissues of normal and obese mice. Furthermore, the knockdown of Drd4 upregulated the expression of brown adipocyte-specific genes and proteins while downregulating lipogenesis and the adipogenesis marker proteins. Drd4 silencing also upregulated the expression of key signaling molecules involved in ATP-dependent thermogenesis in both cells. This was further elucidated by mechanistic studies showing that a Drd4 knockdown mediates UCP1-dependent thermogenesis via the cAMP/PKA/p38MAPK pathway in 3T3-L1 adipocytes and UCP1-independent thermogenesis via the cAMP/SLN/SERCA2a pathway in C2C12 muscle cells. In addition, siDrd4 also mediates myogenesis via the cAMP/PKA/ERK1/2/Cyclin D3 pathway in C2C12 muscle cells. Silencing of Drd4 promotes β3-AR-dependent browning in 3T3-L1 adipocytes and α1-AR/SERCA-based thermogenesis through an ATP-consuming futile process in C2C12 muscle cells. Understanding the novel functions of DRD4 on adipose and muscle tissues in terms of its ability to enhance energy expenditure and regulate whole-body energy metabolism will aid in developing novel obesity intervention techniques.








Similar content being viewed by others
Data availability
All supporting data and materials are included in this manuscript.
Change history
04 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00424-023-02819-7
Abbreviations
- ABHD5/Abhd5 :
-
Abhydrolase domain containing 5 encoding gene
- ACC:
-
Acyl-CoA carboxylase
- ACOX:
-
Acyl-coenzyme A oxidase 1
- AMPK:
-
AMP-activated protein kinase
- ATF2:
-
Activating transcription factor 2
- ATGL:
-
Adipose triglyceride lipase
- AR:
-
Adrenergic receptor
- CD36/Cd36 :
-
Cluster of differentiation 36 protein/encoding gene
- C/EBP:
-
CCAAT/enhancer-binding protein
- CKmt:
-
Mitochondrial creatine kinase
- COX-4:
-
Cyclooxygenase-4
- CPT1:
-
Carnitine palmitoyltransferase 1
- CYT-C:
-
Cytochrome C
- Ccnd3 :
-
Gene encoding G1/S-specific cyclin-D3
- CREB:
-
cAMP-response element binding protein
- CaMKII:
-
Calcium/calmodulin-dependent protein kinase II
- DR:
-
Dopamine receptor
- DRD4:
-
Dopamine receptor D4
- ERK:
-
Extracellular signal-regulated kinase
- FAS:
-
Fatty acid synthase
- HSL:
-
Hormone-sensitive lipase
- MYH/Myh :
-
Myosin heavy chain/encoding gene
- MYOD:
-
Myoblast determination protein
- MYOG/Myog :
-
Myogenin/encoding gene
- Nrf1 :
-
Nuclear respiratory factor 1 encoding gene
- PGC-1α:
-
Peroxisome proliferator-activated receptor-gamma coactivator 1α
- p38 MAPK:
-
p38 mitogen-activated protein kinase
- PKA:
-
Protein kinase A
- PPAR:
-
Peroxisome proliferator-activated receptor
- PRDM16:
-
PR domain containing 16
- RyR/Ryr :
-
Ryanodine receptor/encoding gene
- Slc27 :
-
Solute carrier family 27 encoding fatty acid transporter
- SERCA:
-
Sarco/endoplasmic reticulum Ca2+-ATPase
- UCP:
-
Uncoupling protein
References
Chouchani ET, Kazak L, Spiegelman BM (2017) Mitochondrial reactive oxygen species and adipose tissue thermogenesis: bridging physiology and mechanisms. J Biol Chem 292:16810–16816. https://doi.org/10.1074/jbc.R117.789628
Thyagarajan B, Foster MT (2017) Beiging of white adipose tissue as a therapeutic strategy for weight loss in humans. Horm Mol Biol Clin Investig 31:1–13. https://doi.org/10.1515/hmbci-2017-0016
Pan R, Zhu X, Maretich P, Chen Y (2020) Combating obesity with thermogenic fat: Current challenges and advancements. Front Endocrinol 11:185. https://doi.org/10.3389/fendo.2020.00185
Chang SH, Song NJ, Choi JH, Yun UJ, Park KW (2019) Mechanisms underlying UCP1 dependent and independent adipocyte thermogenesis. Obes Rev 20:241–251. https://doi.org/10.1111/obr.12796
Ikeda K, Yamada T (2020) UCP1 dependent and independent thermogenesis in Brown and Beige Adipocytes. Front Endocrinol 11:1–6. https://doi.org/10.3389/fendo.2020.00498
Fuller-Jackson JP, Henry BA (2018) Adipose and skeletal muscle thermogenesis: studies from large animals. J Endocrinol 237:R99–R115. https://doi.org/10.1530/JOE-18-0090
Cox B, Ennis C, Lee TF (1981) The function of dopamine receptors in the central thermoregulatory pathways of the rat. Neuropharmacolog 20:1047–1051. https://doi.org/10.1016/0028-3908(81)90095-2
Lee TF, Mora F, Myers RD (1985) Dopamine and thermoregulation: an evaluation with special reference to dopaminergic pathways. Neurosci Biobehav Rev 9:589–598. https://doi.org/10.1016/0149-7634(85)90005-3
Ögren SO, Fuxe K (1988) Apomorphine and pergolide induce hypothermia by stimulation of dopamine D-2 receptors. Acta Physiol Scand 133:91–95. https://doi.org/10.1111/j.1748-1716.1988.tb08384.x
Im D, Inoue A, Fujiwara T, Nakane T, Yamanaka Y, Uemura T, Mori C, Shiimura Y, Kimura KT, Asada H, Nomura N, Tanaka T, Yamashita A, Nango E, Tono K, Kadji FMN, Aoki J, Iwata S, Shimamura T (2020) Structure of the dopamine D2 receptor in complex with the antipsychotic drug spiperone. Nat Commun 11:1–11. https://doi.org/10.1038/s41467-020-20221-0
Yu J, Zhu J, Deng J, Shen J, Du F, Wu X, Chen Y, Li M, Wen Q, Xiao Z, Zhao Y (2022) Dopamine receptor D1 signaling stimulates lipolysis and browning of white adipocytes. Biochem Biophys Res Commun 588:83–89. https://doi.org/10.1016/j.bbrc.2021.12.040
Kohlie R, Perwitz N, Resch J, Schmid SM, Lehnert H, Klein J, Iwen KA (2017) Dopamine directly increases mitochondrial mass and thermogenesis in brown adipocytes. J Mol Endocrinol 58:57–66. https://doi.org/10.1530/JME-16-0159
Chipkin RE (1988) Effects of D1 and D2 antagonists on basal and apomorphine decreased body temperature in mice and rats. Pharmacol Biochem Behav 30:683–686. https://doi.org/10.1016/0091-3057(88)90084-6
Folgueira C, Beiroa D, Porteiro B, Duquenne M, Puighermanal E, Fondevila MF, Barja-Fernández S, Gallego R, Hernández-Bautista R, Castelao C, Senra A (2019) Hypothalamic dopamine signalling regulates brown fat thermogenesis. Nat Metab 1:811–829. https://doi.org/10.1038/s42255-019-0099-7
Brizuela M, Antipov A, Blessing WW, Ootsuka Y (2019) Activating dopamine D2 receptors reduces brown adipose tissue thermogenesis induced by psychological stress and by activation of the lateral habenula. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-019-56125-3
Tavares G, Martins FO, Melo BF, Matafome P, Conde SV (2021) Peripheral dopamine directly acts on insulin-sensitive tissues to regulate insulin signaling and metabolic function. Front Pharmacol 12:713418. https://doi.org/10.3389/fphar.2021.713418
Tavares G, Marques D, Barra C, Rosendo-Silva D, Costa A, Rodrigues T, Matafome P (2021) Dopamine D2 receptor agonist, bromocriptine, remodels adipose tissue dopaminergic signalling and upregulates catabolic pathways, improving metabolic profile in type 2 diabetes. Mol Metab 51:101241. https://doi.org/10.1016/j.molmet.2021.101241
Cincotta AH, Meier AH, Cincotta MJ (1999) Bromocriptine improves glycaemic control and serum lipid profile in obese type 2 diabetic subjects: a new approach in the treatment of diabetes. Expert Opin Investig Drugs 8:1683–1707. https://doi.org/10.1517/13543784.8.10.1683
Aslanoglou D, Bertera S, Sánchez-Soto M, Benjamin Free R, Lee J, Zong W, Xue X, Shrestha S, Brissova M, Logan RW, Wollheim CB, Trucco M, Yechoor VK, Sibley DR, Bottino R, Freyberg Z (2021) Dopamine regulates pancreatic glucagon and insulin secretion via adrenergic and dopaminergic receptors. Transl Psychiatry 11:59. https://doi.org/10.1038/s41398-020-01171-z
Caravaggio F, Borlido C, Hahn M, Feng Z, Fervaha G, Gerretsen P, Nakajima S, Plitman E, Chung JK, Iwata Y, Wilson A, Remington G, Graff-Guerrero A (2015) Reduced insulin sensitivity is related to less endogenous dopamine at D2/3 receptors in the ventral striatum of healthy nonobese humans. Int J Neuropsychopharmacol 18:1–10. https://doi.org/10.1093/ijnp/pyv014
Zeng C, Han Y, Huang H, Yu C, Ren H, Shi W, He D, Huang L, Yang C, Wang X, Zhou L, Jose PA (2009) D1-like receptors inhibit insulin-induced vascular smooth muscle cell proliferation via down-regulation of insulin receptor expression. J Hypertens 27:1033–1041. https://doi.org/10.1097/HJH.0b013e3283293c7b
Yu C, Wang Z, Han Y, Liu Y, Wang WE, Chen C, Wang H, Jose PA, Zeng C (2014) Dopamine D4 receptors inhibit proliferation and migration of vascular smooth muscle cells induced by insulin via down-regulation of insulin receptor expression. Cardiovasc Diabetol 13:1–11. https://doi.org/10.1186/1475-2840-13-97
Reichart DL, Hinkle RT, Lefever FR, Dolan ET, Dietrich JA, Sibley DR, Isfort RJ (2011) Activation of the dopamine 1 and dopamine 5 receptors increase skeletal muscle mass and force production under non-atrophying and atrophying conditions. BMC Musculoskelet Disord 12:27. https://doi.org/10.1186/1471-2474-12-27
Zhang Y, Ren H, Lu X, He D, Han Y, Wang H, Zeng C, Shi W (2016) Inhibition of D4 dopamine receptors on insulin receptor expression and effect in renal proximal tubule cells. J Am Heart Assoc 5:1–12. https://doi.org/10.1161/JAHA.115.002448
Neve KA, Seamans JK, Trantham-Davidson H (2004) Dopamine receptor signaling. J Recept Signal Transduct Res 24:165–205. https://doi.org/10.1081/RRS-200029981
Lei S (2014) Cross interaction of dopaminergic and adrenergic systems in neural modulation. Int J Physiol Pathophysiol Pharmacol 6:137
Mund RA, Frishman WH (2013) Brown adipose tissue thermogenesis: β3 adrenoreceptors as a potential target for the treatment of obesity in humans. Cardiol Rev 21:265–269. https://doi.org/10.1097/CRD.0b013e31829cabff
Evans BA, Merlin J, Bengtsson T, Hutchinson DS (2019) Adrenoceptors in white, brown, and brite adipocytes. Br J Pharmacol 176:2416–2432. https://doi.org/10.1111/bph.14631
Dang TTH, Choi M, Pham HG, Yun JW (2021) Cytochrome P450 2F2 (CYP2F2) negatively regulates browning in 3T3-L1 white adipocytes. Eur J Pharmacol 908:174318. https://doi.org/10.1016/j.ejphar.2021.174318
Haddish K, Yun JW (2022) L-dihydroxyphenylalanine (L-Dopa) induces brown-like phenotype in 3T3-L1 white adipocytes via activation of dopaminergic and β3-adrenergic receptors. Biotechnol Bioproc Eng 27:792–806. https://doi.org/10.1007/s12257-021-0361-1
Haddish K, Yun JW (2022) Dopaminergic and adrenergic receptors synergistically stimulate browning in 3T3-L1 white adipocytes. J Physiol Biochem in press. https://doi.org/10.1007/s13105-022-00928-y
John P, Konhilas YL (2015) AMP-Activated protein kinase signaling in cancer and cardiac hypertrophy. Cardiovasc Pharm Open Access 4:154. https://doi.org/10.4172/2329-6607.1000154
Wang D, Ji X, Liu J, Li Z, Zhang X (2018) Dopamine receptor subtypes differentially regulate autophagy. Int J Mol Sci 19:1540. https://doi.org/10.3390/ijms19051540
Yang W, Munhall AC, Johnson SW (2019) AMP-activated protein kinase slows D2 dopamine autoreceptor desensitization in substantia nigra neurons. Neuropharmacology 158:107705. https://doi.org/10.1016/j.neuropharm.2019.107705
Fricke K, Heitland A, Maronde E (2004) Cooperative activation of lipolysis by protein kinase A and protein kinase C pathways in 3T3-L1 adipocytes. Endocrinology 145:4940–4947. https://doi.org/10.1210/en.2004-0803
Wang X, Zhong P, Yan Z (2002) Dopamine D4 receptors modulate GABAergic signaling in pyramidal neurons of prefrontal cortex. J Neurosci 22:9185–9193. https://doi.org/10.1523/JNEUROSCI.22-21-09185.2002
Han SF, Jiao J, Zhang W, Xu JY, Zhang W, Fu CL, Qin LQ (2017) Lipolysis and thermogenesis in adipose tissues as new potential mechanisms for metabolic benefits of dietary fiber. Nutrition 33:118–124. https://doi.org/10.1016/j.nut.2016.05.006
Liu X, Guo Y, Yang Y, Qi C, Xiong T, Chen Y, Wu G, Zeng C, Wang D (2021) DRD4 (Dopamine D4 Receptor) mitigate abdominal aortic aneurysm via decreasing p38 MAPK (mitogen-activated protein kinase)/NOX4 (NADPH Oxidase 4) axis-associated oxidative stress. Hypertension 78:294–307. https://doi.org/10.1161/HYPERTENSIONAHA.120.16738
Du M, Perry RL, Nowacki NB, Gordon JW, Salma J, Zhao J, Aziz A, Chan J, Siu KM, McDermott JC (2008) Protein kinase A represses skeletal myogenesis by targeting myocyte enhancer factor 2D. Mol Cell Biol 28:2952–2970. https://doi.org/10.1128/MCB.00248-08
Chen AE, Ginty DD, Fan CM (2005) Protein kinase A signaling via CREB controls myogenesis induced by Wnt proteins. Nature 433:317–322. https://doi.org/10.1038/nature03126
Jiang W, Zhu J, Zhuang X, Zhang X, Luo T, Esser KA, Ren H (2015) Lipin1 regulates skeletal muscle differentiation through extracellular signal-regulated kinase (ERK) activation and cyclin D complex-regulated cell cycle withdrawal. J Biol Chem 290:23646–23655. https://doi.org/10.1074/jbc.M115.686519
Dolma S, Selvadurai HJ, Lan X, Lee L, Kushida M, Voisin V, Whetstone H, So M, Aviv T, Park N, Zhu X, Xu CJ, Head R, Rowland KJ, Bernstein M, Clarke ID, Bader G, Harrington L, Brumell JH, Dirks PB (2016) Inhibition of dopamine receptor D4 impedes autophagic flux, proliferation, and survival of glioblastoma stem cells. Cancer Cell 29:859–873. https://doi.org/10.1016/j.ccell.2016.05.002
Mizuta K, Zhang Y, Xu D, Mizuta F, D’Ovidio F, Masaki E, Emala CW (2013) The dopamine D1 receptor is expressed and facilitates relaxation in airway smooth muscle. Respir Res 14:1–14. https://doi.org/10.1186/1465-9921-14-89
Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M, Shinoda K, Chen Y, Lu X, Maretich P, Tajima K, Ajuwon KM, Soga T, Kajimura S (2017) UCP1-independent signaling involving SERCA2b mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med 23:1454–1465. https://doi.org/10.1038/nm.4429
Periasamy M, Maurya SK, Sahoo SK, Singh S, Reis FCG, Bal NC (2017) Role of SERCA pump in muscle thermogenesis and metabolism. Compr Physiol 7:879–890. https://doi.org/10.1002/cphy.c160030
Pant M, Bal NC, Periasamy M (2016) Sarcolipin: a key thermogenic and metabolic regulator in skeletal muscle. Trends Endocrinol Metab 27:881–892. https://doi.org/10.1016/j.tem.2016.08.006
Servan-Schreiber D, Printz H, Cohen JD (1990) A network model of catecholamine effects: gain, signal-to-noise ratio, and behavior. Science 249:892–895. https://doi.org/10.1126/science.2392679
Ciruela F, Canela L, Burgueño J, Soriguera A, Cabello N, Canela EI, Casadó V, Cortés A, Mallol J, Woods AS, Ferré S (2005) Heptaspanning membrane receptors and cytoskeletal/scaffolding proteins: focus on adenosine, dopamine, and metabotropic glutamate receptor function. J Mol Neurosci 26:277–329. https://doi.org/10.1385/jmn:26:2-3:277
De Bartolomeis A, Tomasetti C (2012) Calcium-dependent networks in dopamine–glutamate interaction: the role of postsynaptic scaffolding proteins. Mol Neurobiol 46:275–296. https://doi.org/10.1007/s12035-012-8293-6
Funding
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT, No. 2019R1A2C2002163).
Author information
Authors and Affiliations
Contributions
Kiros Haddish performed the experimental design, conducted experiments, analyzed the data, performed the statistical analysis, and wrote the manuscript; and Jong Won Yun carried out scientific support, critically reviewed the manuscript and experimental design, and approved the manuscript version to be published.
Corresponding author
Ethics declarations
Ethical approval
All the procedures were performed according to the guidelines approved by the National Institutes of Health. All animal experiments were approved by the Committee for Laboratory Animal Care and Use of Daegu University (DUIACC-2022–001-0310–001).
Consent to participate
Not applicable.
Consent for publication
All authors have seen and approved the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The original article published with incorrect Figure 5. The replacement figure has been missed during correction stage.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haddish, K., Yun, J.W. Dopamine receptor D4 (DRD4) negatively regulates UCP1- and ATP-dependent thermogenesis in 3T3-L1 adipocytes and C2C12 muscle cells. Pflugers Arch - Eur J Physiol 475, 757–773 (2023). https://doi.org/10.1007/s00424-023-02816-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-023-02816-w