Abstract
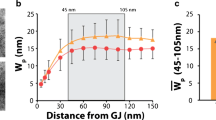
Several studies have disagreed on measurements of cardiac conduction velocity (CV) in mice with a heterozygous knockout of the connexin gene Gja1—a mutation that reduces the gap junction (GJ) protein, Connexin43 (Cx43), by 50 %. We noted that perfusate ionic composition varied between studies and hypothesized that extracellular ionic concentration modulates CV dependence on GJs. CV was measured by optically mapping wild-type (WT) and heterozygous null (HZ) hearts serially perfused with solutions previously associated with no change (Solution 1) or CV slowing (Solution 2). In WT hearts, CV was similar for Solutions 1 and 2. However, consistent with the hypothesis, Solution 2 in HZ hearts slowed transverse CV (CVT) relative to Solution 1. Previously, we showed CV slowing in a manner consistent with ephaptic conduction correlated with increased perinexal inter-membrane width (W P) at GJ edges. Thus, W P was measured following perfusion with systematically adjusted [Na+]o and [K+]o in Solutions 1 and 2. A wider W P was associated with reduced CVT in WT and HZ hearts, with the greatest effect in HZ hearts. Increasing [Na+]o increased CVT only in HZ hearts. Increasing [K+]o slowed CVT in both WT and HZ hearts with large W P but only in HZ hearts with narrow W P. Conclusion: When perinexi are wide, decreasing excitability by modulating [Na+]o and [K+]o increases CV sensitivity to reduced Cx43. By contrast, CV is less sensitive to Cx43 and ion composition when perinexi are narrow. These results are consistent with cardiac conduction dependence on both GJ and non-GJ (ephaptic) mechanisms.






Similar content being viewed by others
References
Agullo-Pascual E, Lin X, Leo-Macias A, Zhang M, Liang FX, Li Z, Pfenniger A, Lubkemeier I, Keegan S, Fenyo D, Willecke K, Rothenberg E, Delmar M (2014) Super-resolution imaging reveals that loss of the C-terminus of connexin43 limits microtubule plus-end capture and NaV1.5 localization at the intercalated disc. Cardiovasc Res 104(2):371–381. doi:10.1093/cvr/cvu195
Ballantyne F 3rd, Davis LD, Reynolds EW Jr (1975) Cellular basis for reversal of hyperkalemic electrocardiographic changes by sodium. Am J Physiol 229(4):935–940
Bayly PV, KenKnight BH, Rogers JM, Hillsley RE, Ideker RE, Smith WM (1998) Estimation of conduction velocity vector fields from epicardial mapping data. IEEE Trans Biomed Eng 45(5):563–571. doi:10.1109/10.641337
Beauchamp P, Choby C, Desplantez T, de Peyer K, Green K, Yamada KA, Weingart R, Saffitz JE, Kleber AG (2004) Electrical propagation in synthetic ventricular myocyte strands from germline connexin43 knockout mice. Circ Res 95(2):170–178. doi:10.1161/01.RES.0000134923.05174.2f
Cascio WE, Yang H, Muller-Borer BJ, Johnson TA (2005) Ischemia-induced arrhythmia: the role of connexins, gap junctions, and attendant changes in impulse propagation. J Electrocardiol 38(4 Suppl):55–59. doi:10.1016/j.jelectrocard.2005.06.019
Celes MR, Torres-Duenas D, Alves-Filho JC, Duarte DB, Cunha FQ, Rossi MA (2007) Reduction of gap and adherens junction proteins and intercalated disc structural remodeling in the hearts of mice submitted to severe cecal ligation and puncture sepsis. Crit Care Med 35(9):2176–2185
Chitaev NA, Troyanovsky SM (1998) Adhesive but not lateral E-cadherin complexes require calcium and catenins for their formation. J Cell Biol 142(3):837–846
Danik SB, Liu F, Zhang J, Suk HJ, Morley GE, Fishman GI, Gutstein DE (2004) Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ Res 95(10):1035–1041. doi:10.1161/01.RES.0000148664.33695.2a
Dhillon PS, Gray R, Kojodjojo P, Jabr R, Chowdhury R, Fry CH, Peters NS (2013) Relationship between gap-junctional conductance and conduction velocity in mammalian myocardium. Circ Arrhythm Electrophysiol 6(6):1208–1214. doi:10.1161/CIRCEP.113.000848
Eloff BC, Lerner DL, Yamada KA, Schuessler RB, Saffitz JE, Rosenbaum DS (2001) High resolution optical mapping reveals conduction slowing in connexin43 deficient mice. Cardiovasc Res 51(4):681–690
Ganote CE, Grinwald PM, Nayler WG (1984) 2,4-Dinitrophenol (DNP)-induced injury in calcium-free hearts. J Mol Cell Cardiol 16(6):547–557
Gettes LS, Reuter H (1974) Slow recovery from inactivation of inward currents in mammalian myocardial fibres. J Physiol 240(3):703–724
Greve G, Rotevatn S, Saetersdal T, Oksendal AN, Jynge P (1985) Ultrastructural studies of intercalated disc separations in the rat heart during the calcium paradox. Res Exp Med Z Gesamte Exp Med Einschliesslich Exp Chir 185(3):195–206
Guerrero PA, Schuessler RB, Davis LM, Beyer EC, Johnson CM, Yamada KA, Saffitz JE (1997) Slow ventricular conduction in mice heterozygous for a connexin43 null mutation. J Clin Invest 99(8):1991–1998. doi:10.1172/JCI119367
Kagiyama Y, Hill JL, Gettes LS (1982) Interaction of acidosis and increased extracellular potassium on action potential characteristics and conduction in guinea pig ventricular muscle. Circ Res 51(5):614–623
Kleber AG, Rudy Y (2004) Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev 84(2):431–488. doi:10.1152/physrev.00025.2003
Lin J, Keener JP (2014) Microdomain effects on transverse cardiac propagation. Biophys J 106(4):925–931. doi:10.1016/j.bpj.2013.11.1117
Lurtz MM, Louis CF (2007) Intracellular calcium regulation of connexin43. Am J Physiol Cell Physiol 293(6):C1806–C1813. doi:10.1152/ajpcell.00630.2006
Morley GE, Vaidya D, Samie FH, Lo C, Delmar M, Jalife J (1999) Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping. J Cardiovasc Electrophysiol 10(10):1361–1375
Nygren A, Giles WR (2000) Mathematical simulation of slowing of cardiac conduction velocity by elevated extracellular. Ann Biomed Eng 28(8):951–957
Poelzing S, Rosenbaum DS (2004) Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol 287(4):H1762–H1770. doi:10.1152/ajpheart.00346.2004
Research Animals Resources UoM (2009) Reference values for laboratory animals. Normal hematology values. www.ahc.umn.edu/rar/refvalues.html. 2013
Rhett JM, Ongstad EL, Jourdan J, Gourdie RG (2012) Cx43 associates with Na (v)1.5 in the cardiomyocyte perinexus. J Membr Biol 245(7):411–422. doi:10.1007/s00232-012-9465-z
Sato PY, Musa H, Coombs W, Guerrero-Serna G, Patino GA, Taffet SM, Isom LL, Delmar M (2009) Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circ Res 105(6):523–526. doi:10.1161/CIRCRESAHA.109.201418
Seidel T, Salameh A, Dhein S (2010) A simulation study of cellular hypertrophy and connexin lateralization in cardiac tissue. Biophys J 99(9):2821–2830. doi:10.1016/j.bpj.2010.09.010
Spach MS, Heidlage JF, Dolber PC, Barr RC (2000) Electrophysiological effects of remodeling cardiac gap junctions and cell size: experimental and model studies of normal cardiac growth. Circ Res 86(3):302–311
Thomas SA, Schuessler RB, Berul CI, Beardslee MA, Beyer EC, Mendelsohn ME, Saffitz JE (1998) Disparate effects of deficient expression of connexin43 on atrial and ventricular conduction: evidence for chamber-specific molecular determinants of conduction. Circulation 97(7):686–691
Thomas SP, Kucera JP, Bircher-Lehmann L, Rudy Y, Saffitz JE, Kleber AG (2003) Impulse propagation in synthetic strands of neonatal cardiac myocytes with genetically reduced levels of connexin43. Circ Res 92(11):1209–1216. doi:10.1161/01.RES.0000074916.41221.EA
Toure A, Cabo C (2010) Effect of cell geometry on conduction velocity in a subcellular model of myocardium. IEEE Trans Biomed Eng 57(9):2107–2114. doi:10.1109/TBME.2010.2050064
Vaidya D, Tamaddon HS, Lo CW, Taffet SM, Delmar M, Morley GE, Jalife J (2001) Null mutation of connexin43 causes slow propagation of ventricular activation in the late stages of mouse embryonic development. Circ Res 88(11):1196–1202
van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, Jongsma HJ, Opthof T, de Bakker JM (2004) Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation 109(8):1048–1055. doi:10.1161/01.CIR.0000117402.70689.75
Veeraraghavan R, Lin J, Hoeker G, Keener J, Gourdie R, Poelzing S (2015) Sodium channels in the Cx43 gap junction perinexus may constitute a cardiac ephapse: an experimental and modeling study. Pflugers Arch
Veeraraghavan R, Salama ME, Poelzing S (2012) Interstitial volume modulates the conduction velocity-gap junction relationship. Am J Physiol Heart Circ Physiol 302(1):H278–H286. doi:10.1152/ajpheart.00868.2011
Watt FM, Mattey DL, Garrod DR (1984) Calcium-induced reorganization of desmosomal components in cultured human keratinocytes. J Cell Biol 99(6):2211–2215
Weidmann S (1955) The effect of the cardiac membrane potential on the rapid availability of the sodium-carrying system. J Physiol 127(1):213–224
Yanagi N, Maruyama T, Uehata S, Wakimoto Y, Sasaki Y, Arita M (1998) Electrical and mechanical abnormalities in the heart of a schizophrenic patient with hyponatremia derived from water intoxication. J Cardiol 32(3):197–204
Acknowledgments
We would like to thank Dr. Jeffrey E. Saffitz at Harvard Medical School for generously providing us with the Cx43+/− mice and Drs. Robert Price and Jeffrey Davis at the University of South Carolina and Kathy Lowe at Virginia Tech for their assistance with electron microscopy. This work was supported in part by grants from the National Institutes of Health (R01 HL102298-01A1 to SP, R01 HL56728-10A2 to RGG, R01-1DE019355-01 RGG subcontract) and Virginia Tech Carilion Research Institute Medical Research Scholars Award to SG.
Conflict of interest
RGG holds stock in FirstString Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
George, S.A., Sciuto, K.J., Lin, J. et al. Extracellular sodium and potassium levels modulate cardiac conduction in mice heterozygous null for the Connexin43 gene. Pflugers Arch - Eur J Physiol 467, 2287–2297 (2015). https://doi.org/10.1007/s00424-015-1698-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-015-1698-0