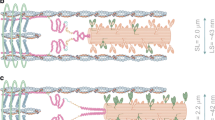
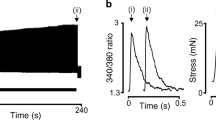
Abstract
Myocardium generates power to perform external work on the circulation; yet, many questions regarding intermolecular mechanisms regulating power output remain unresolved. Power output equals force × shortening velocity, and some interesting new observations regarding control of these two factors have arisen. While it is well established that sarcomere length tightly controls myocyte force, sarcomere length–tension relationships also appear to be markedly modulated by PKA-mediated phosphorylation of myofibrillar proteins. Concerning loaded shortening, historical models predict independent cross-bridge mechanics; however, it seems that the mechanical state of one population of cross-bridges affects the activity of other cross-bridges by, for example, recruitment of cross-bridges from the non-cycling pool to the cycling force-generating pool during submaximal Ca2+ activation. This is supported by the findings that Ca2+ activation levels, myofilament phosphorylation, and sarcomere length are all modulators of loaded shortening and power output independent of their effects on force. This fine tuning of power output probably helps optimize myocardial energetics and to match ventricular supply with peripheral demand; yet, the discernment of the chemo-mechanical signals that modulate loaded shortening needs further clarification since power output may be a key convergent point and feedback regulator of cytoskeleton and cellular signals that control myocyte growth and survival.


Similar content being viewed by others
References
Ait Mou Y, le Guennac J-Y, Mosca E, de Tombe PP, Cazorla O (2008) Differential contribution of cardiac sarcomeric proteins in the myofibrillar force response to stretch. Pflugers Arch 457:25–36
Cazorla O, Le Guennec J-Y, White E (2000) Length-tension relationships of subepicardial and sub-endocardial single ventricular myocytes from rat to ferret hearts. J Mol Cell Cardiol 32:735–744
Chiu YC, Ballou EW, Ford LE (1987) Force, velocity, and power changes during normal and potentiated contractions of cat papillary muscle. Circ Res 60:446–458
Colson BA, Bekyarova T, Fitzsimons DP, Irving TC, Moss RL (2007) Radial displacement of myosin cross-bridges in mouse myocardium due to ablation of myosin binding-C. J Mol Biol 367:36–41
Colson BA, Bekyarova T, Locher MR, Fitzsimons DP, Irving TC, Moss RL (2008) Protein kinase A-mediated phosphorylation of cMyBP-C increases proximity of myosin heads to actin in resting myocardium. Circulation Research 103:244
Daniels M, Noble MIM, Ter Keurs HEDJ, Wohlfart B (1984) Velocity of sarcomere shortening in rat cardiac muscle: relationship to force, sarcomere length, calcium and time. J Physiol 355:367–381
De Tombe PP, ter Keurs EDJ (1992) An internal viscous element limits unloaded velocity of sarcomere shortening in rat cardiac trabeculae. J Physiol 454:619–642
Edman KAP (1979) The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibers. J Physiol 291:143–159
Endoh M, Blinks JR (1988) Actions of sympathomimetic amines on the Ca2+ transients and contractions of rabbit myocardium: reciprocal changes in myofibrillar responsiveness to Ca2+ mediated through- and ß-adrenoceptors. Circ Res 62:247–265
Fabiato A, Fabiato F (1975) Dependence of the contractile activation of skinned cardiac cells on the sarcomere length. Nature 256:54–56
Ford LE (1991) Mechanical manifestations of activation of cardiac muscle. Circ Res 68:621–637
Ford LE, Huxley AF, Simmons RM (1985) Tension transients during steady shortening of frog muscle fibres. J Physiol 361:131–150
Ford LE, Nakagawa K, Desper J, Seow CY (1991) Effect of osmotic compression on force-velocity properties of glycerinated rabbit skeletal muscle cells. J Gen Physiol 97:73–88
Fuchs F, Martyn DA (2005) Length-dependent Ca2+ activation in cardiac muscle: some remaining questions. J Muscle Res Cell Motil 26:199–212
Gordon AM, Huxley AF, Julian FJ (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibers. J Physiol 184:170–192
Gordon AM, Homsher E, Regnier M (2000) Regulation of contraction in striated muscle. Physiol Rev 80:853–924
Granzier HLM, Burns DH, Pollack GH (1989) Sarcomere length dependence of the force-velocity relation in single frog muscle fibers. Biophys J 55:499–507
Guyton AC, Jones CE, Coleman TG (1973) Circulatory physiology: cardiac output and its regulation. Saunders, Philadelphia
Hanft LM, McDonald KS (2009) Sarcomere length dependence of power output is increased after PKA treatment in rat cardiac myocytes. Am J Physiol 296:H1524–H1531
Hanft LM, McDonald KS (2010) Length dependence of force generation exhibit similarities between rat cardiac myocytes and skeletal muscle fibres. J Physiol 588:2891–2903
Hanft LM, McDonald KS (2010) Determinants of loaded shortening in rat cardiac myocytes, 2010 biophysical society meetings abstract
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7:255–318
Julian FJ (1971) The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol 218:117–145
Kentish JC, ter Keurs HEDJ, Noble MI, Ricciardi I, Schouten VJA (1983) The relationships between force, [Ca2+] and sarcomere length in skinned trabeculae from rat ventricle. J Physiol 345:24P
Konhilas JP, Irving TC, Wolska BM, Jweied EE, Martin AF, Solaro RJ, deTombe PP (2003) Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing. J Physiol 547:951–961
Korte FS, McDonald KS (2007) Sarcomere length dependence of rat skinned cardiac myocyte mechanical properties: dependence on myosin heavy chain. J Physiol 581:725–739
Korte FS, McDonald KS, Harris SP, Moss RL (2003) Loaded shortening, power output, and rate of force redevelopment are increased with knockout of cardiac myosin binding protein-C. Circ Res 93:752–758
Luther PK, Bennett PM, Knupp C, Craig R, Padron R, Harris SP, Patel J, Moss RL (2008) Understanding the organisation and role of myosin binding protein C in normal striated muscle by comparison with MyBP-C knockout cardiac muscle. J Mol Biol 384:60–72
Martyn DA, Rondinone JF, Huntsman LL (1983) Myocardial segment velocity at a low load: time, length, and calcium dependence. Am J Physiol 13:H708–H714
McDonald KS (2000) Ca2+ dependence of loaded shortening in rat skinned cardiac myocytes and skeletal muscle fibers. J Physiol 525:169–181
McDonald KS, Moss RL (2000) Strongly binding myosin cross-bridges regulate loaded shortening and power output in cardiac myocytes. Circ Res 87:768–773
McKillop DF, Geeves MA (1993) Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J 65:693–701
Moss RL (1992) Ca2+ regulation of mechanical properties of striated muscle: mechanistic studies using extraction and replacement of regulatory proteins. Circ Res 70:865–884
Moss RL, Allen JD, Greaser ML (1986) Effects of partial extraction of troponin complex upon the tension-pCa relation in rabbit skeletal muscle. Further evidence that tension development involves cooperative effects within the thin filament. J Gen Physiol 87:761–774
Piazzesi G, Reconditi M, Linari M, Lucii L, Bianco P, Brunello E, Decostre V, Stewart A, Gore DB, Irving TC, Irving M, Lombardi V (2007) Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell 131:784–795
Podolin RA, Ford LE (1983) The influence of calcium on shortening velocity of skinned frog muscle cells. J Muscle Res Cell Motil 4:263–282
Podolsky RJ, Teichholz LE (1970) The relation between calcium and contraction kinetics in skinned muscle fibres. J Physiol 211:19–35
Sarnoff SJ (1955) Myocardial contractility as described by ventricular function curves; observations on Starling’s law of the heart. Physiol Rev 35:107–122
Sweitzer NK, Moss RL (1993) Determinants of loaded shortening velocity in single cardiac myocytes permeabilized with alpha-hemolysin. Circ Res 73:1150–1162
ter Keurs HE, Rijnsburger WH, van Heuningen R, Nagelsmit MJ (1980) Tension development and sarcomere length in rat cardiac trabeculae. Evidence of length-dependent activation. Circ Res 46:703–714
Tobacman L (1996) Thin filament-mediated regulation of cardiac contraction. Annu Rev Physiol 58:447–481
van der Velden J, de Jong JW, Owen VJ, Burton PBJ, Stienen GJM (2000) Effect of protein kinase A on calcium sensitivity of force and its sarcomere length dependence in human cardiomyocytes. Cardiovasc Res 46:487–495
Vibert P, Craig R, Lehman W (1997) Steric-model for activation of muscle thin filaments. J Mol Biol 266:8–14
Wahr PA, Metzger JM (1999) Role of Ca2+ and cross-bridges in skeletal muscle thin filament activation probed by Ca2+ sensitizers. Biophys J 76:2166–2176
Wolska BM, Stojanovic MO, Luo W, Kranias EG, Solaro RJ (1996) Effect of ablation of phospholamban on dynamics of cardiac myocyte contraction and intracellular calcium. Am J Physiol 271:C391–C397
Xu C, Craig R, Tobacman L, Horowitz R, Lehman W (1999) Tropomyosin positions in regulated thin filaments revealed by cryoelectron microscopy. Biophys J 77:985–992
Acknowledgments
Special acknowledgment is given to Dr. Laurin M. Hanft for helpful comments regarding all aspects of the manuscript and with preparation of the figures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McDonald, K.S. The interdependence of Ca2+ activation, sarcomere length, and power output in the heart. Pflugers Arch - Eur J Physiol 462, 61–67 (2011). https://doi.org/10.1007/s00424-011-0949-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-011-0949-y