Abstract
This study examines the effects of the intracellular protein FKBP12.6 on action potential and associated K+ currents in isolated adult rabbit ventricular cardiomyocytes. FKBP12.6 was over-expressed by ~6 times using a recombinant adenovirus coding for human FKBP12.6. This over-expression caused prolongation of action potential duration (APD) by ~30%. The amplitude of the transient outward current (I to) was unchanged, but rate of inactivation at potentials positive to +40 mV was increased. FKBP12.6 over-expression decreased the amplitude of the inward rectifier current (I K1) by ~25% in the voltage range −70 to −30 mV, an effect prevented by FK506 or lowering intracellular [Ca2+] below 1 nM. Over-expression of an FKBP12.6 mutant, which cannot bind calcineurin, prolonged APD and affected I to and I K1 in a similar manner to wild-type protein. These data suggest that FKBP12.6 can modulate APD via changes in I K1 independently of calcineurin binding, suggesting that FKBP12.6 may affect APD by direct interaction with I K1.
Similar content being viewed by others
Introduction
The development of the immunosuppressant drug FK506 resulted in the identification of a family of intracellular proteins termed FK506 binding proteins (FKBP) [12]. The 12.6-kDa member of this family (FKBP12.6) binds to and inhibits the enzyme calcineurin, a Ca2+/calmodulin-dependent phosphatase [19]. The FK506-FKBP12.6 complex cannot bind to calcineurin; thus, FK506 treatment will dis-inhibit the enzyme. In mammalian heart muscle, FKBP12.6 also binds to and modulates the activity of the ryanodine receptor type 2 (RyR2), a Ca2+ channel in the sarcoplasmic reticulum (SR) [20]. FKBP12.6 is thought to play an important role in modulating the process of Ca2+-induced Ca2+ release from the SR [14, 15], a key process in excitation–contraction (E–C) coupling. Modulation of RyR2 activity by FKBP12.6 is not via effects on calcineurin; instead, the effects are thought to be via direct interaction of the two proteins [21]. But RyR2 may not be the only cellular target for FKBP12.6 in heart cells. Indirect evidence for alternative targets comes from studies showing that FK506 treatment prolongs the action potential duration (APD) in isolated rat cardiomyocytes [7, 9]. This effect is a consequence of the inhibition of a number of currents including transient outward current (I to), delayed rectifier (I K) and inward rectifier (I K1) [8, 9]. Although these studies suggest a link between FKBP12.6 and K+ currents in the heart, the work with FK506 cannot distinguish between two options: (1) a direct effect of the drug on the channels or (2) an effect mediated via FKBP. This study examines the effects of FKBP12.6 over-expression in adult rabbit cardiomyocytes on the action potential, the inwardly rectifying potassium current (I K1) and the transient outward current (I to). The data indicate that FKBP12.6 can regulate I K1 in the physiological voltage range in a manner that is independent of calcineurin binding.
Materials and methods
Isolation of adult ventricular rabbit cardiomyocytes
Adult rabbit ventricular cardiomyocytes were isolated from New Zealand White rabbits by standard enzymatic dissociation as described previously [16]. In brief, animals were anaesthetised using sodium pentobarbital (100 mg/kg) with 1,000 IU heparin. The aorta was rapidly cannulated after the heart was removed and perfused in Langendorff mode with a Ca2+-free Krebs solution (pH 7.4 with NaOH) consisting of (mM): 120.0 NaCl, 20.0 4-2-hydroxyethyl-1-piperazineethanesulfonic acid (HEPES), 5.4 KCl, 0.52 NaH2PO4, 3.5 MgCl2⋅6H20, 20.0 taurine, 10.0 creatine, 11.1 glucose. After sufficient perfusion, the Ca2+-free Krebs was switched to Krebs containing 0.05 mM Ca2+ plus collagenase and protease for 5.5 min. Finally, perfusion was changed to a 1% bovine serum albumin 0.075 mM Ca2+ Krebs solution. The digested left ventricle was removed from the cannula, cut into small pieces and agitated in 0.125 mM Ca2+ solution for 1 h. Consequently, the myocytes were re-suspended in a series of Krebs solution containing (mM) 0.25, 0.5 and 1 Ca2+.
Adenoviral transfection and cell culture
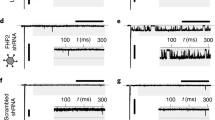
Recombinant adenoviruses were generated using standard procedures [16]. Briefly, a human heart muscle-specific cDNA sample was used to clone full-length cDNA of the human FKBP12.6 gene (Fig. 4a) by using polymerase chain reaction (PCR) and PCR primers to span the coding region of FKBP12.6 cDNA. Three populations of adenovirus-transfected myocytes were cultured to overexpress: (1) β-galactosidase (Ad-LacZ), (2) FKBP12.6 (Ad-FKBP21.6) and (3) a mutant form of FKBP12.6 (Ad-FKBP12.6 m) that cannot bind calicneurin (see below). Myocytes were transfected with a multiplicity of infection (MOI) of 100. Transfected myocytes were subsequently incubated in a supplemented medium, M199 (Sigma), for 24 h, at 37°C. Verification of FKBP12.6 expression and virus transfection efficiency has been detailed elsewhere [14, 16]. Previous measurements suggest that the level of FKBP12.6 over-expression was approximately 6× normal values (Fig. 4b (i)) [16].
Mutant FKBP12.6
The FKBP12.6 mutant was constructed by the site-directed mutagenesis of the wild-type FKBP12.6, resulting in a G89P V90K mutant and the deletion of the calcineurin binding site (Fig. 4a). The mutant was tested for protein and DNA levels (Fig. 4b (ii) and c). The full-length FKBP12.6 m cDNA sequence was inserted downstream from a cytomegalovirus promoter into vector pACCMV·pLpA, and recombination with vector pJM17 was performed in HEK293 cells. The production, purification and titration of adenovirus containing the FKBP12.6 gene (Ad-FKBP12.6) were performed according to standard procedures [1].
Solutions
The isolated cardiomyocytes were allowed to settle on the base of a recording chamber mounted on the stage of an inverted microscope and superfused with a HEPES-based Krebs–Heinseleit solution consisting of (mM): 144.0 NaCl, 5.4 KCl, 0.3 NaH2P04, 1.0 MgCl2, 5.0 HEPES, 11.1 glucose and 1.8 CaCl2, (pH 7.4 with NaOH). For I K1 measurement, pipettes were filled with an intracellular solution of composition (mM): 15 K aspartate (DL), 10 KCl, 10 HEPES, 4.5 MgCl2, 4 Na2ATP, 1 Na2CrP, 25 K2–ethyleneglycoltetraacetic acid (EGTA), 25 CaK2EGTA , free [Ca2+] 170 nM, pH 7.25. In some experiments, 10 mM K2EGTA was used (free [Ca2+] <1 nM). For I to measurement, pipettes were filled with an intracellular solution of composition (mM): 120 K aspartate (DL), 20 KCl, 5 HEPES, 4 Na2ATP, 1 MgCl2, 1 EGTA (pH 7.25 with KOH). To block various channels, the following compounds were used in the superfusate: cadmium chloride at 0.3 mM to block the calcium-sensitive component of I to; barium chloride at 0.2 mM to block the inward rectifier (I K1) and TTX (Tetrodotoxin) at 3 × 10−5 M to block I Na. Thapsigargin (5 μM) was included in the superfusate for a sub-set of I K1 measurements. For action potential measurement, pipettes were filled with an intracellular solution of composition (mM): 120 KCl, 10 NaCl, 10 HEPES and 0.1 EGTA (pH 7.25). Microelectrodes were pulled from boroscilicate glass (Clark Electromedical, Oxford, UK), fire polished, and filled with the appropriate internal solution when filled pipette resistance measured 3–6 MΩ.
Electrophysiological protocols
Action potentials were elicited in current clamp mode using a square pulse of 1 nA and 5 ms duration at 0.5 Hz. The action potential duration was measured at 10%, 50% and 90% of the repolarisation. In separate experiments, membrane currents were measured in discontinuous (switch) voltage clamp mode using the whole cell patch technique with an Axoclamp 2A amplifier (Axon Instruments, Foster City, CA, USA). Protocols were controlled, recorded and analysed using a PC and Axon Laboratory Software (Axon Instruments).
Ito is activated in response to depolarising steps and comprises two components: the 4-AP-sensitive K+ current and the Ca2+-sensitive chloride current [10]. In this study, the Ca2+-sensitive chloride component was blocked using 0.3 mM Cd2+ [10]. Transient outward current (Ito) was studied by holding at a membrane potential of −80 mV followed by a step to −40 mV for 50 ms in addition to TTX to completely inactivate INa (Fig. 2a (i)). Ten millivolts depolarising steps from −50 to +70 mV were applied for 500 ms duration at 0.1 Hz. Ito was measured as the difference between peak Ito and the sustained outward current at the end of the pulse [11, 13, 22]. Pharmacological interventions to block Ito were not used in this study since the effects are more wide ranging than exclusively to Ito [17]. It has also been shown that the steady state current at the end of the pulse is not affected by 4-AP [3]. The inward rectifier (IK1) was studied by using a holding potential of −80 mV with 10 mV steps from −100 to +100 mV for 750 ms duration at 0.2 Hz in the absence and then in the presence of 0.25 mM Ba2+ [6]. IK1 was taken as the Ba2+-sensitive current.
Results
Action potential duration
Action potentials in response to brief current injections were recorded in isolated rabbit cardiomyocytes from both Ad-FKBP12.6 and Ad-LacZ groups. Figure 1a (i) shows typical action potentials recorded from myocytes at 19–21°C stimulated at 0.5 Hz by brief current injections. As the average data in Fig. 1b show, the duration of the action potential in cardiomyocytes over-expressing FKBP12.6 was increased by approximately 33% at both 90% of repolarisation (APD90) and 50% of repolarisation (APD50), but there were no significant effects at 10% of repolarisation (APD10). The time from the stimulus until the repolarisation phase reached zero millivolts (0 mV) was 173 ± 17 ms (n = 12) in Lac-Z transfected cells and 213 ± 26 ms (n = 10) in cells over-expressing FKBP12.6, but this difference did not reach significance. Thus, the major affects on action potential difference appeared to emerge in the repolarisation phase beyond approximately 0 mV. Neither the resting membrane potential nor the maximum rate of depolarisation was significantly different between experimental groups (data not shown).
Effect of FKBP12.6 over-expression on action potential duration. a Individual action potentials recorded from single cardiomyocytes after transfection with Ad-LacZ (black line) and Ad-FKBP12.6 (grey line). b Average action potential duration at 90% (i), 50% (ii) and 10% (iii) repolarisation from Ad-LacZ (n = 12) and Ad-FKBP12.6 (n = 10) groups. **P < 0.01, *P < 0.05
Transient outward current (I to)
Figure 2a (i) shows a typical I to voltage record from a myocyte transfected with Ad-LacZ. Figure 2a (ii) and (iii) show typical I to records from cells expressing AdLacZ and AdFKBP12.6, respectively. The average I to amplitude in the Ad-LacZ and Ad-FKBP12.6 myocyte groups is shown in Fig. 2b (i). Over-expression of FKBP12.6 did not cause a significant change in peak I to or the amplitude of the transient component of I to. The small remaining steady-state component was increased in FKBP12.6 over-expression by ~63% across the voltage range (e.g. 0.0019 nA/pF vs. 0.0031 nA/pF at 30 mV P < 0.05). The rate constant of decay of the current was measured by fitting an exponential decay to >80% of the current decay between peak and steady state. The value of the rate constant increased (Fig. 2b (ii)) at more polarised potentials. FKBP12.6 over-expression resulted in a significantly enhanced rate of decay at membrane potentials more positive than +40 mV (48.4% increase at 50 mV, 48.2% at 60 mV and 49.6% at 70 mV; Fig. 2b (ii)).
Effect of FKBP12.6 over-expression on the transient outward current (I to). a A typical family of voltage (i) and current recordings from a single Ad-LacZ cardiomyocyte (ii) and Ad-FKBP12.6 cardiomyocyte (iii). b (i) current–voltage relationship in Ad-LacZ (open circles; n = 19) and Ad-FKBP12.6 (closed circles; n = 20). (ii) Rate constant of decay of I to in Ad-LacZ (open circles, n = 19) and Ad-FKBP12.6 (closed circles, n = 20). *P < 0.05
Inward rectifier (I K1 )
Figure 3a (i) shows a typical I K1voltage record from a myocyte transfected with Ad-LacZ. Figure 3a (ii) and (iii) show representative traces of Ba2+-sensitive currents in an Ad-LacZ and FKBP12.6 over-expressing cell, respectively. The current displays the typical pronounced inward rectification that is characteristic of I K1. The average I K1 currents over the range −80 mV to 0 mV for both the Ad-LacZ and Ad-FKBP12.6 groups are shown in Fig. 3b (i). FKBP12.6 over-expression significantly decreased the magnitude of I K1 in the voltage range −70 to −30 mV. These measurements were made using a pipette solution containing ~170 nM Ca2+, buffered with 50 mM EGTA to ensure constant and standardised Ca2+ levels between experimental groups. The specificity of the effect of transfection with Ad-FKBP12.6 was supported by the observation that incubation with 10 μM FK-506 inhibited the effect of FKBP12.6 over-expression (Fig. 3b (ii)). To investigate the Ca2+ sensitivity of the effects of FKBP12.6 over-expression, intracellular [Ca2+] was lowered to <1 nM by inclusion of (10 mM EGTA, no added Ca2+) in the patch pipette solution. As shown in Fig. 3b (iii), reducing the cytoplasmic [Ca2+] abolished the effect of FKBP12.6 over-expression on I K1. To investigate the role of SR Ca2+ release on I K1, cardiomyocytes were perfused with the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA2A) blocker Thapsigargin (~170 nM Ca2+) to deplete the SR of Ca2+. As illustrated in Fig. 3b (iv), depleting the SR Ca2+ had no effect on the magnitude of I K1.
Effect of FKBP12.6 over-expression on the inward rectifier (I K1). b A typical family of voltage (i) and current recordings from a single Ad-LacZ (ii) and a single Ad-FKBP12.6 cardiomyocyte (iii). b (i) Average current–voltage relationships for cardiomyocytes from Ad-LacZ (open circles, n = 10) and Ad-FKBP12.6 groups (closed circles, n = 11). The patch pipette solution was buffered at 170 nM Ca2+ with 50 mM EGTA. (ii) Average current–voltage relationships for cardiomyocytes from Ad-LacZ (open circles, n = 10) and Ad-FKBP12.6 (closed circle, n = 12) on incubation with 10 μM FK-506. (iii) Average current–voltage relationships for cardiomyocytes from Ad-LacZ (open circles, n = 10) and Ad-FKBP12.6 groups (closed circles, n = 11) recorded using a pipette solution containing <1 nM Ca2+. (iv) Average current−voltage relationships for cardiomyocytes perfused with normal perfusate (~170 nM Ca2+; closed squares, n = 6) and perfusate containing 5 μM Thapsigargin (closed triangles, n = 6). **P < 0.05
To investigate the role of calcineurin in this response, a mutant form of FKBP12.6 was generated (Fig. 4a) that was unable to bind to calcineurin yet retained other properties of the protein [18]. An adenovirus expressing FKBP12.6 m was created (Ad-FKBP12.6 m) and tested for protein levels (Fig. 4b (ii)) and DNA (Fig. 4c). As with the wild-type protein, over-expression of the mutant form of the protein caused a prolongation of the action potential (APD90: 376 ± 19 ms (n = 12) vs. 681 ± 32 ms (n = 15), Ad-LacZ vs. Ad-FKBP12.6 m). As shown in Fig. 5a, the effects of Ad-FKBP12.6 m transfection did not significantly alter I to amplitude, whilst the rate constant for decay showed a non-significant trend to increase over the physiological range of membrane voltages. Over-expression of the mutant form of FKBP12.6 inhibited I K1 (Fig. 5b (i)) in a similar fashion to the wild-type protein. FKBP12.6 m over-expression significantly decreased the magnitude of I K1 in the voltage range −70 to −10 mV. This effect was inhibited by prior incubation with the drug FK506 (Fig. 5b (ii)), and the effect was absent when intracellular [Ca2+] was lowered to <1 nM (Fig. 5b (iii)).
FKBP12.6 and FKBP12.6 mutant. a Amino acid sequence of human FKBP12.6 and indication of the insertion of the mutation made to the calcineurin binding site. b Western immunoblot analysis of (i) Ad-LacZ (LacZ)-infected and Ad-FKBP12.6-GFP (FKBP12.6)-infected myocytes and (ii) Ad-FKBP12.6 m-GFP (FKBP12.6)-infected myocytes. Cells were infected with indicated MOI and harvested after 48 h of culture time. FKBP12.6 protein expression increases with increasing virus titres. The fact that FKBP12.6 migrates as a broad band during electrophoresis and could not be clearly separated from endogenous FKBP12 is most likely attributable to the lysis procedure of the cells under high-salt conditions. b RT-PCR analysis of Ad-FKBP12.6 m infected myocytes. Cells infected at indicated MOI and harvested 48 h post transfection. (+) denotes positive control with FKBP12.6 plasmid DNA as template
Effect of FKBP12.6 mutant over-expression on I to and I K1. a (i) Average I to current–voltage relationships for cardiomyocytes from Ad-LacZ (open circles, n = 10) and Ad-FKBP12.6 m groups (closed circles, n = 11). (ii) Rate constant of decay of I to from Ad-LacZ (open circles, n = 10) and Ad-FKBP12.6 m (closed circle, n = 11). b (i) Average I K1 current–voltage relationships for cardiomyocytes from Ad-LacZ (open circles, n = 13) and Ad-FKBP12.6 m groups (closed circles, n = 13) recorded using a pipette solution containing 170 nM Ca2+. (ii) Average I K1 current–voltage relationships for cardiomyocytes from Ad-LacZ (open circles, n = 13) and Ad-FKBP12.6 m groups (closed circles, n = 13) recorded upon incubation with FK506. (iii) Average I K1 current–voltage relationships for cardiomyocytes from Ad-LacZ (open circles, n = 10) and Ad-FKBP12.6 m groups (closed circles, n = 11) recorded using a pipette solution containing <1 nM Ca2+. **P < 0.01, ***P < 0.001
Discussion
In this study, the effects of over-expression of FKBP12.6 on the action potential and two of the underlying K+ currents were studied in isolated adult rabbit cardiomyocytes. Over-expression of FKBP12.6 prolonged the action potential significantly. The differences between action potentials recorded from the two groups showed significant differences in the latter phases of the repolarisation more negative to 0 mV. Previous work has shown that neither I Ca,L nor NCX (Sodium-Calcium exchanger) are affected by acute upregulation of FKBP12.6 in the intact cardiomyocyte [14]; therefore, modulation of K+ currents is the most likely explanation for the effect.
Effects of FKBP12.6 over-expression on I to
FKBP12.6 over-expression does not have significant effects on the peak current, the amplitude of the transient component or the voltage dependence of I to However, the small remaining steady-state component was significantly increased in cells over-expressing FKBP12.6. At very positive potentials (>+40 mV), the rate of inactivation after FKBP12.6 expression was enhanced compared to the control values. I to is active during phase 1 of the cardiac action potential, and drug-induced reduction of I to is known to prolong action potential duration [2]. A more rapid inactivation (as observed after FKBP12.6 over-expression) might be expected to prolong the action potential duration, but this link has not been reported. Furthermore, the effect of FKBP12.6 on I to inactivation was observed at potentials more positive than +40 mV, i.e. beyond the range of voltages experienced normally during an action potential. In this study, action potential duration was not significantly prolonged at 0 mV on the repolarisation phase. Significant prolongation was observed at 50% of repolarisation (approximately −20 mV) and 90% repolarisation (approximately −70 mV). These potentials are considerably more negative than the potentials at which I to were affected. Therefore, it is unlikely that the changes in I to are responsible for the observed changes in action potential duration.
Effects of FKBP12.6 over-expression on I K1
The shape and magnitude of I K1 current–voltage relationship shown in this study is similar to those published previously [4, 6]. FKBP12.6 over-expression caused a significant decrease of approximately 25% in the amplitude of I K1 within the voltage range of −70 to −30 mV. At potentials positive to 0 mV, I K1 currents were not significantly different from zero in both experimental groups. The conditions under which this decrease in I K1 amplitude was observed (170 nM Ca2+, 50 mM EGTA) would prevent FKBP12.6 induced changes in RyR2 activity that would alter the intracellular Ca2+ both in the bulk cytoplasm and also locally next to the sarcolemma. This indicates that RyR2 is not involved in the action of FKBP12.6 on I K1. This is further supported by the lack of effect upon depleting the SR of Ca2+ using Thapsigargin, suggesting that the effect of FKBP12.6 over-expression is not via modulation of RyR2 activity.
The effects of FKBP12.6 over-expression were abolished by incubation with the drug FK506. This supports the view that the effect of FKBP12.6 over-expression was due to a specific interaction. The magnitude of I K1 in both Ad-LacZ and Ad-FKBP12.6 groups between −60 mV and −30 mV was on average smaller in the presence of FK506, by ~50% (Ad-LacZ group) and ~45% (FKBP12.6 over-expression group; P < 0.05 at −30 mV). FK506-mediated depression of I K1 was previously reported in rat cardiomyocytes [9]. This result appears counter-intuitive since FKBP12.6 over-expression decreased I K1, removal of FKBP12.6 with FK506 would be expected to increase I K1 (i.e. the opposite of the observed effects). Therefore, the depressive effect of FK506 on I K1 may result from an action of the drug on I K1 independent of FKBP12.6. This effect appears to dominate over the enhancement of I K1 as a result of FKBP12.6 removal.
The mode of action of FKBP12.6 on I K1 is not known; one possibility is that this effect is mediated by phosphorylation of I K1 via inhibition of calcineurin [5]. However, a decrease in I K1 was evident in experiments involving the mutant form of FKBP12.6 (Fig. 5) indicating that calcineurin was not involved in the effect. It is however interesting to note that the magnitude of the effect of FKBP12.6 m on action potential duration and I K1 was much larger than the native protein. Whilst the conditions of culture with both adenoviruses was the same, the over-expression of the two forms of the protein was not quantified with sufficient precision to be confident that this effect was not simply the result of higher intracellular levels of the mutant protein. Alternatively, the mutation could alter the relative affinity of FKBP12.6 for the target protein; further work is required to distinguish these two options.
Removal of Ca2+ from the cytosol significantly increased I K1 and abolished the ability of FKBP12.6 to modulate I K1. Data indicating an inhibitory effect of intracellular Ca2+ on I K1 have previously been reported [23], but this modulating influence has not been extensively studied. The molecular basis for the Ca2+ dependency of the action of FKBP12.6 deserves further investigation. Both FK506 and low Ca2+ appear to acutely prevent the ability of FKBP12.6 to affect I K1. This suggests that FKBP12.6 modulates I K1 via changes in the biophysical properties of the channel (including the Ca2+ sensitivity), rather than ion channel expression.
In summary, this paper shows for the first time that FKBP12.6 over-expression decreases I K1 in a Ca2+-dependent and FK506-sensitive fashion. No major effects of FKBP12.6 were observed on the I to amplitude. This suggests a role for this cytoplasmic protein in addition to the known effects on the RyR2. Thus, altered expression and binding of FKBP12.6, as is known to occur in heart failure [15], may alter E–C coupling indirectly via changes in the action potential duration in addition to effects on the RyR2.
References
Becker TC, Noel RJ, Coats WS, Gomezfoix AM, Alam T, Gerard RD et al (1994) Use of recombinant adenovirus for metabolic engineering of mammalian-cells. Methods Cell Biol 43:161–189
Berger F, Borchard U, Gelhaar R, Hafner D, Weis TM (1995) Inhibition of pacemaker current by the bradycardic agent ZD 7288 is lost use-dependently in sheep cardiac Purkinje fibres. Naunyn Schmiedebergs Arch Pharmacol 353:64–72
Bogdanov KY, Spurgeon HA, Vinogradova TM, Lakatta EG (1998) Modulation of the transient outward current in adult rat ventricular myocytes by polyunsaturated fatty acids. Am J Physiol-Heart Circ Physiol 43:H571–H579
Bouchard R, Clark RB, Juhasz AE, Giles WR (2004) Changes in extracellular K + concentration modulate contractility of rat and rabbit cardiac myocytes via the inward rectifier K + current I-K1. J Physiol-Lond 556:773–790
Bram RJ, Hung DT, Martin PK, Schreiber SL, Crabtree GR (1993) Identification of the immunophilins capable of mediating inhibition of signal-transduction by Cyclosporine-A and Fk506 - Roles of calcineurin binding and cellular location. Mol Cell Biol 13:4760–4769
Cordeiro JM, Spitzer KW, Giles WR (1998) Repolarizing K+ currents in rabbit heart Purkinje cells. J Physiol 508(Pt 3):811–823
duBell WH, Lederer WJ, Rogers TB (2000) K(+) currents responsible for repolarization in mouse ventricle and their modulation by FK-506 and rapamycin. Am J Physiol Heart Circ Physiol 278:H886–H897
duBell WH, Wright PA, Lederer WJ, Rogers TB (1997) Effect of the immunosupressant FK506 on excitation-contraction coupling and outward K + currents in rat ventricular myocytes. J Physiol 501(Pt 3):509–516
Fauconnier J, Lacampagne A, Rauzier JM, Fontanaud P, Frapier JM, Sejersted OM et al (2004) Frequency-dependent and proarrhythmogenic effects of FK506 in rat ventricular cells. Am J Physiol Heart Circ Physiol
Hiraoka M, Kawano S (1989) Calcium-sensitive and insensitive transient outward current in rabbit ventricular myocytes. J Physiol-Lond 410:187–212
Hulme JT, Orchard CH (2000) Effect of acidosis on transient outward potassium current in isolated rat ventricular myocytes. Am J Physiol-Heart Circ Physiol 278:H50–H59
Kino T, Hatanaka H, Miyata S, Inamura N, Nishiyama M, Yajima T et al (1987) FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot (Tokyo) 40:1256–1265
Komukai K, Brette F, Yamanushi TT, Orchard CH (2002) K + current distribution in rat sub-epicardial ventricular myocytes. Pflugers Archiv-European Journal Of Physiology 444:532–538
Loughrey CM, Seidler T, Miller SL, Prestle J, MacEachern KE, Reynolds DF et al (2004) Over-expression of FK506-binding protein FKBP12.6 alters excitation-contraction coupling in adult rabbit cardiomyocytes. J Physiol 556:919–934
Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N et al (2000) PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101:365–376
Prestle J, Janssen PM, Janssen AP, Zeitz O, Lehnart SE, Bruce L et al (2001) Overexpression of FK506-binding protein FKBP12.6 in cardiomyocytes reduces ryanodine receptor-mediated Ca(2+) leak from the sarcoplasmic reticulum and increases contractility. Circ Res 88:188–194
Scamps F (1996) Characterization of a beta-adrenergically inhibited K + current in rat cardiac ventricular cells. J Physiol-Lond 491:81–97
Seidler T, Kania A, Prestle J, Wagner S, Koegler H, Hasenfuss G (2002) Mutation of the Calcineurin Binding Site in the 12.6 kda FK-506 Binding Protein (FKBP12.6) Leads to Loss of the FKBP12.6 mediated increase in myocardial contractile performance. Circulation 106(19 Suppl):II1=766
Thomson AW, Bonham CA, Zeevi A (1995) Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther Drug Monit 17:584–591
Timerman AP, Onoue H, Xin HB, Barg S, Copello J, Wiederrecht G et al (1996) Selective binding of FKBP12.6 by the cardiac ryanodine receptor. J Biol Chem 271:20385–20391
Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D et al (2004) Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science 304:292–296
Wettwer E, Amos GJ, Posival H, Ravens U (1994) Transient outward current in human ventricular myocytes of subepicardial and subendocardial origin. Circ Res 75:473–482
Zaza A, Rocchetti M, Brioschi A, Cantadori A, Ferroni A (1998) Dynamic Ca2+-induced inward rectification of K+ current during the ventricular action potential. Circ Res 82:947–956
Acknowledgements
This study was funded by the Wellcome Trust Foundation (SK) and the Deutsche Forschungsgemeinschaft (TS). Thanks to Mrs. Anne Ward and Mrs. Aileen Rankin for their technical contributions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kettlewell, S., Seidler, T. & Smith, G.L. The effects of over-expression of the FK506-binding protein FKBP12.6 on K+ currents in adult rabbit ventricular myocytes. Pflugers Arch - Eur J Physiol 458, 653–660 (2009). https://doi.org/10.1007/s00424-009-0666-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-009-0666-y