Abstract
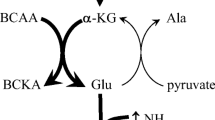
It was proposed that a contraction-induced increase in tricarboxylic acid cycle intermediates (TCAI) is obligatory for the increase in muscle oxygen uptake at the start of exercise. To test this hypothesis, we measured changes in muscle TCAI during the initial seconds of intense exercise and used dichloroacetate (DCA) in an attempt to alter the level of TCAI. Five men performed strenuous leg kicking exercise (64±8 W) under noninfused control (CON) and DCA-supplemented conditions; biopsies (vastus lateralis) were obtained at rest and after 5, 15, and 180 s of exercise. In CON, the total concentration of three measured TCAI (ΣTCAI: citrate, malate, and fumarate) increased (p<0.05) by 71% during the first 15 s of exercise. The ΣTCAI was lower (p<0.05) in DCA than in CON at rest [0.18±0.02 vs 0.64±0.09 mmol kg−1 dry weight (d.w.)], after 5 s (0.30±0.07 vs 0.85±0.14 mmol kg−1 d.w.), and 15 s of exercise (0.60±0.07 vs 1.09±0.16 mmol kg−1 d.w.), but not different after 3 min (3.12±0.53 vs 3.23±0.55 mmol kg−1 d.w.). Despite differences in the level of muscle TCAI, muscle phosphocreatine degradation was similar in DCA and CON during the first 15 s of exercise (17.5±3.3 vs 25.6±4.1 mmol kg−1 d.w.). Taken together with our previous observation that DCA does not alter muscle oxygen uptake during the initial phase of intense leg kicking exercise (Bangsbo et al. Am J Physiol 282:R273–R280, 2002), the present data suggest that muscle TCAI accumulate during the initial seconds of exercise; however, this increase is not essential for the contraction-induced increase in mitochondrial respiration.



Similar content being viewed by others
References
Andersen P, Adams RP, Sjøgaard G, Thorboe A, Saltin B (1985) Dynamic knee extension as a model for the study of an isolated exercising muscle in man. J Appl Physiol 59:1647–1653
Bangsbo J, Gollnick PD, Graham TE, Juel C, Kiens B, Mizuno M, Saltin B (1990) Anaerobic energy production and O2 deficit–debt relationship during exhaustive exercise in humans. J Physiol 422:539–559
Bangsbo J, Krustrup P, González-Alonso J, Saltin B (2000) Muscle oxygen kinetics at onset of intense dynamic exercise in humans. Am J Physiol 279(3):R899–R906
Bangsbo J, Krustrup P, González-Alonso J, Saltin B (2001) ATP production and mechanical efficiency of human skeletal muscle during intense exercise: effect of previous exercise. Am J Physiol 280:E956–E964
Bangsbo J, Gibala M, Krustrup P, González-Alonso J, Saltin B (2002) Enhanced pyruvate dehydrogenase activity does not affect muscle O2 uptake at onset of intense exercise in humans. Am J Physiol 282:R273–R280
Bergmeyer HU (1974) Methods of enzymatic analysis, 2nd edn. Academic, New York
Bergström J (1962) Muscle electrolytes in man. Scand J Clin Lab Invest Suppl 68:1–101
Chung Y, Mole PA, Sailasuta N, Tran TK, Hurd R, Jue T (2005) Control of respiration and bioenergetics during muscle contraction. Am J Physiol 288(3):C730–C738
Constantin-Teodosiu D, Simpson EJ, Greenhaff PL (1998) Effects of pyruvate, dichloroacetate and adrenaline infusion on pyruvate dehydrogenase complex activation and tricarboxylic acid cycle intermediates in human skeletal muscle. J Physiol 506:102P
Dawson KD, Howarth KR, Tarnopolsky MA, Wong ND, Gibala MJ (2003) Short-term training attenuates muscle TCA cycle expansion during exercise in women. J Appl Physiol 95(3):999–1004
Dawson KD, Baker DJ, Greenhaff PL, Gibala MJ (2005) An acute decrease in TCA cycle intermediates does not affect aerobic energy delivery in contracting rat skeletal muscle. J Physiol 565(2):637–643
Gibala MJ, Saltin B (1999) PDH activation by dichloroacetate reduces TCA cycle intermediates at rest but not during exercise in humans. Am J Physiol 277(1):E33–E38
Gibala MJ, Tarnopolsky MA, Graham TE (1997) Tricarboxylic acid cycle intermediates in human muscle at rest and during prolonged cycling. Am J Physiol 272(1):E239–E244
Gibala MJ, MacLean DA, Graham T, Saltin B (1998) Tricarboxylic acid cycle intermediate pool size and estimated cycle flux in human muscle during exercise. Am J Physiol 275(2):E235–E242
Gibala MJ, Young ME, Taegtmeyer H (2000) Anaplerosis of the citric acid cycle: role in energy metabolism of heart and skeletal muscle. Acta Physiol Scand 168(4):657–665
Gibala MJ, González-Alonso J, Saltin B (2002) Dissociation between muscle tricarboxylic acid cycle pool size and aerobic energy provision during prolonged exercise in humans. J Physiol 545(2):705–713
Grassi B (2000) Skeletal muscle VO2 on-kinetics: set by O2 delivery or by O2 utilization? New insights into an old issue. Med Sci Sports Exerc 32(1):108–116
Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD (1996) Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol 80:988–998
Grassi B, Gladden LB, Samaja M, Stary CM, Hogan MC (1998) Faster adjustment of O2 delivery does not affect VO2 on-kinetics in isolated in situ canine muscle. J Appl Physiol 85(4):1394–1403
Grassi B, Gladden LB, Stary CM, Wagner PD, Hogan MC (1998) Peripheral O2 diffusion does not affect VO2 on-kinetics in isolated in situ canine muscle. J Appl Physiol 85(4):1404–1412
Harris RC, Hultman E, Nordesjö O (1974) Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Scand J Clin Lab Invest 33:109–120
Howarth KR, LeBlanc PJ, Heigenhauser GJ, Gibala MJ (2004) Effect of endurance training on muscle TCA cycle metabolism during exercise in humans. J Appl Physiol 97(2):579–584
Howlett RA, Heigenhauser GJ, Spriet LL (1999) Skeletal muscle metabolism during high-intensity sprint exercise is unaffected by dichloroacetate or acetate infusion. J Appl Physiol 87(5):1747–1751
Hughson RL, Shoemaker JK, Tschakovsky ME, Kowalchuk (1996) Dependence of muscle VO2 on blood flow of forearm exercise. J Appl Physiol 81(4):1516–1521
Hughson RL, Tschakovsky ME, Houston ME (2001) Regulation of oxygen consumption at the onset of exercise. Exerc Sport Sci Rev 29(3):129–133
Korzeniewski B, Zoladz JA (2004) Factors determining the oxygen consumption rate (VO2) on-kinetics in skeletal muscles. Biochem J 379(3):703–710
Krustrup P, González-Alonso J, Quistorff B, Bangsbo J (2001) Muscle heat production and anaerobic energy turnover during repeated intense dynamic exercise in humans. J Physiol 536:947–956
Krustrup P, Hellsten Y, Bangsbo J (2004) Intense interval training enhances human skeletal muscle oxygen uptake in the initial phase of dynamic exercise at high but not at low intensities. J Physiol 559(1):335–345
Lee SH, Davis EJ (1979) Carboxylation and decarboxylation reactions. Anaplerotic flux and removal of citrate cycle intermediates in skeletal muscle. J Biol Chem 254(2):420–430
Ludvik B, Mayer G, Stifter S, Putz D, Barnas U, Graf H (1993) Effects of dichloroacetate on exercise performance in healthy volunteers. Pflugers Arch 423(3–4):251–254
Messer JI, Jackman MR, Willis WT (2004) Pyruvate and citric acid cycle carbon requirements in isolated skeletal muscle mitochondria. Am J Physiol 286:C565–C572
Molé PA, Chung Y, Tran TK, Sailasuta N, Hurd R, Jue T (1999) Myoglobin desaturation with exercise intensity in human gastrocnemius muscle. Am J Physiol 277(1):R173–R180
Passonneau JV, Lowry OH (1993) Enzymatic analysis: a practical guide. Humana Press, Totowa, NJ
Sahlin K, Katz A, Broberg S (1990) Tricarboxylic acid cycle intermediates in human muscle during prolonged exercise. Am J Physiol 259:C834–C841
Stacpoole PW (1989) The pharmacology of dichloroacetate. Metabolism 38(11):1124–1144
Timmons JA, Gustafsson T, Sundberg CJ, Jansson E, Greenhaff PL (1998) Muscle acetyl group availability is a major determinant of oxygen deficit in humans during submaximal exercise. Am J Physiol 274:E377–E380
Timmons JA, Poucher SM, Constantin-Teodosiu D, Macdonald IA, Greenhaff PL (1998) Regulation of skeletal muscle carbohydrate oxidation during steady-state contraction. Am J Physiol 274:R1384–R1389
Vicini P, Kushmerick MJ (2000) Cellular energetics analysis by a mathematical model of energy balance: estimation of parameters in human skeletal muscle. Am J Physiol 279(1):C213–C224
Walsh B, Tonkonogi M, Söderlund K, Hultman E, Saks V, Sahlin K (2001) The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. J Physiol 537(3):971–978
Williamson JR, Cooper RH (1980) Regulation of the citric acid cycle in mammalian systems. FEBS Lett 117:K73–K83 (Suppl)
Acknowledgements
We thank the subjects for their committed participation. We also thank Merete Vannby and Ingelise Kring for excellent technical assistance. The study was supported by a grant from The Danish National Research Foundation (504-14). In addition, support was obtained from Team Denmark and The Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bangsbo, J., Gibala, M.J., Howarth, K.R. et al. Tricarboxylic acid cycle intermediates accumulate at the onset of intense exercise in man but are not essential for the increase in muscle oxygen uptake. Pflugers Arch - Eur J Physiol 452, 737–743 (2006). https://doi.org/10.1007/s00424-006-0075-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-006-0075-4