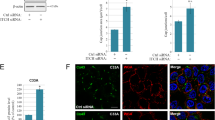
Abstract
c-Jun NH2-terminal protein kinase (JNK) and p38 are stress-activated mitogen-activated protein kinases (MAPK) that are phosphorylated by various stimuli. It has been reported that the loss of desmoglein (DSG) 3, a desmosomal transmembrane core molecule, in keratinocytes impairs cell–cell adhesion accompanied by p38 MAPK activation. To understand the biological role of DSG3 in desmosomes and its relationship with stress-activated MAPKs, we established DSG3 knockout keratinocytes (KO cells). Wild-type cells showed a linear localization of DSG1 to cell–cell contacts, whereas KO cells showed a remarkable reduction despite the increased protein levels of DSG1. Cell–cell adhesion in KO cells was impaired over time, as demonstrated by dispase-based dissociation assays. The linear localization of DSG1 to cell–cell contacts and the strength of cell–cell adhesion were promoted by the pharmacological inhibition of JNK. Conversely, pharmacological activation of JNK, but not p38 MAPK, in wild-type cells reduced the linear localization of DSG1 in cell–cell contacts. Our data indicate that DSG1 and DSG2 in KO cells cannot compensate for the attenuation of cell–cell adhesion strength caused by DSG3 deficiency and that JNK inhibition restores the strength of cell–cell adhesion by increasing the linear localization of DSG1 in cell–cell contacts in KO cells. Inhibition of JNK signaling may improve cell–cell adhesion in diseases in which DSG3 expression is impaired.






Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding authors on reasonable request.
References
Amagai M, Stanley JR (2012) Desmoglein as a target in skin disease and beyond. J Invest Dermatol 132(3 Pt. 2):776–784. https://doi.org/10.1038/jid.2011.390
Amagai M, Fujimori T, Masunaga T, Shimizu H, Nishikawa T, Shimizu N, Takeichi M, Hashimoto T (1995) Delayed assembly of desmosomes in keratinocytes with disrupted classic-cadherin-mediated cell adhesion by a dominant negative mutant. J Invest Dermatol 104(1):27–32. https://doi.org/10.1111/1523-1747.ep12613462
Aono S, Hirai Y (2008) Phosphorylation of claudin-4 is required for tight junction formation in a human keratinocyte cell line. Exp Cell Res 314(18):3326–3339. https://doi.org/10.1016/j.yexcr.2008.08.012
Baron S, Hoang A, Vogel H, Attardi LD (2012) Unimpaired skin carcinogenesis in desmoglein 3 knockout mice. PLoS ONE 7(11):e50024. https://doi.org/10.1371/journal.pone.0050024
Berkowitz P, Hu P, Liu Z, Diaz LA, Enghild JJ, Chua MP, Rubenstein DS (2005) Desmosome signaling. Inhibition of p38MAPK prevents pemphigus vulgaris IgG-induced cytoskeleton reorganization. J Biol Chem 280(25):23778–23784. https://doi.org/10.1074/jbc.M501365200
Berkowitz P, Hu P, Warren S, Liu Z, Diaz LA, Rubenstein DS (2006) P38MAPK inhibition prevents disease in pemphigus vulgaris mice. Proc Natl Acad Sci USA 103(34):12855–12860. https://doi.org/10.1073/pnas.0602973103
Bogoyevitch MA, Court NW (2004) Counting on mitogen-activated protein kinases–ERKs 3, 4, 5, 6, 7 and 8. Cell Signal 16(12):1345–1354. https://doi.org/10.1016/j.cellsig.2004.05.004
Chernyavsky AI, Arredondo J, Kitajima Y, Sato-Nagai M, Grando SA (2007) Desmoglein versus non-desmoglein signaling in pemphigus acantholysis: characterization of novel signaling pathways downstream of pemphigus vulgaris antigens. J Biol Chem 282(18):13804–13812. https://doi.org/10.1074/jbc.M611365200
Delva E, Tucker DK, Kowalczyk AP (2009) The desmosome. Cold Spring Harb Perspect Biol 1(2):a002543. https://doi.org/10.1101/cshperspect.a002543
Garcia MA, Nelson WJ, Chavez N (2018) Cell-cell junctions organize structural and signaling networks. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a029181
Garrod D, Chidgey M (2008) Desmosome structure, composition and function. Biochim Biophys Acta 1778(3):572–587. https://doi.org/10.1016/j.bbamem.2007.07.014
Hagemann C, Blank JL (2001) The ups and downs of MEK kinase interactions. Cell Signal 13(12):863–875. https://doi.org/10.1016/s0898-6568(01)00220-0
Hanakawa Y, Amagai M, Shirakata Y, Sayama K, Hashimoto K (2000) Different effects of dominant negative mutants of desmocollin and desmoglein on the cell-cell adhesion of keratinocytes. J Cell Sci 113(10):1803–1811. https://doi.org/10.1242/jcs.113.10.1803
Hanakawa Y, Matsuyoshi N, Stanley JR (2002) Expression of desmoglein 1 compensates for genetic loss of desmoglein 3 in keratinocyte adhesion. J Invest Dermatol 119(1):27–31. https://doi.org/10.1046/j.1523-1747.2002.01780.x
Hartlieb E, Kempf B, Partilla M, Vigh B, Spindler V, Waschke J (2013) Desmoglein 2 is less important than desmoglein 3 for keratinocyte cohesion. PLoS ONE 8(1):e53739. https://doi.org/10.1371/journal.pone.0053739
Hartlieb E, Rötzer V, Radeva M, Spindler V, Waschke J (2014) Desmoglein 2 compensates for desmoglein 3 but does not control cell adhesion via regulation of p38 mitogen-activated protein kinase in keratinocytes. J Biol Chem 289(24):17043–17053. https://doi.org/10.1074/jbc.M113.489336
Ishii T, Hayakawa H, Igawa T, Sekiguchi T, Sekiguchi M (2018) Specific binding of PCBP1 to heavily oxidized RNA to induce cell death. Proc Natl Acad Sci USA 115(26):6715–6720. https://doi.org/10.1073/pnas.1806912115
Ishikawa S, Nikaido M, Otani T, Ogata K, Iida H, Inai Y, Tamaoki S, Inai T (2022) Inhibition of retinoid X receptor improved the morphology, localization of desmosomal proteins and paracellular permeability in three-dimensional cultures of mouse keratinocytes. Microscopy 71(3):152–160. https://doi.org/10.1093/jmicro/dfac007
Kim JH, Kim SE, Park HS, Lee SH, Lee SE, Kim SC (2019) A homozygous nonsense mutation in the DSG3 gene causes acantholytic blisters in the oral and laryngeal mucosa. J Invest Dermatol 139(5):1187–1190. https://doi.org/10.1016/j.jid.2018.09.038
Kitagawa N, Inai Y, Higuchi Y, Iida H, Inai T (2014) Inhibition of JNK in HaCaT cells induced tight junction formation with decreased expression of cytokeratin 5, cytokeratin 17 and desmoglein 3. Histochem Cell Biol 142(4):389–399. https://doi.org/10.1007/s00418-014-1219-9
Koch PJ, Mahoney MG, Ishikawa H, Pulkkinen L, Uitto J, Shultz L, Murphy GF, Whitaker-Menezes D, Stanley JR (1997) Targeted disruption of the pemphigus vulgaris antigen (desmoglein 3) gene in mice causes loss of keratinocyte cell adhesion with a phenotype similar to pemphigus vulgaris. J Cell Biol 137(5):1091–1102. https://doi.org/10.1083/jcb.137.5.1091
Kojima T, Fuchimoto J, Yamaguchi H, Ito T, Takasawa A, Ninomiya T, Kikuchi S, Ogasawara N, Ohkuni T, Masaki T, Hirata K, Himi T, Sawada N (2010) C-Jun N-terminal kinase is largely involved in the regulation of tricellular tight junctions via tricellulin in human pancreatic duct epithelial cells. J Cell Physiol 225(3):720–733. https://doi.org/10.1002/jcp.22273
Kyriakis JM, Avruch J (2001) Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81(2):807–869. https://doi.org/10.1152/physrev.2001.81.2.807
Lewis JE, Jensen PJ, Wheelock MJ (1994) Cadherin function is required for human keratinocytes to assemble desmosomes and stratify in response to calcium. J Invest Dermatol 102(6):870–877. https://doi.org/10.1111/1523-1747.ep12382690
Lichti U, Anders J, Yuspa SH (2008) Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc 3(5):799–810. https://doi.org/10.1038/nprot.2008.50
Mahoney MG, Wang Z, Rothenberger K, Koch PJ, Amagai M, Stanley JR (1999) Explanations for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris. J Clin Invest 103(4):461–468. https://doi.org/10.1172/JCI5252
Minakami M, Kitagawa N, Iida H, Anan H, Inai T (2015) p38 mitogen-activated protein kinase and c-Jun NH2-terminal protein kinase regulate the accumulation of a tight junction protein, ZO-1, in cell-cell contacts in HaCaT cells. Tissue Cell 47(1):1–9. https://doi.org/10.1016/j.tice.2014.10.001
Naydenov NG, Hopkins AM, Ivanov AI (2009) C-Jun N-terminal kinase mediates disassembly of apical junctions in model intestinal epithelia. Cell Cycle 8(13):2110–2121. https://doi.org/10.4161/cc.8.13.8928
Nikaido M, Otani T, Kitagawa N, Ogata K, Iida H, Anan H, Inai T (2019) Anisomycin, a JNK and p38 activator, suppresses cell-cell junction formation in 2D cultures of K38 mouse keratinocyte cells and reduces claudin-7 expression, with an increase of paracellular permeability in 3D cultures. Histochem Cell Biol 151(5):369–384. https://doi.org/10.1007/s00418-018-1736-z
Pearson LL, Castle BE, Kehry MR (2001) CD40-mediated signaling in monocytic cells: up-regulation of tumor necrosis factor receptor-associated factor mRNAs and activation of mitogen-activated protein kinase signaling pathways. Int Immunol 13(3):273–283. https://doi.org/10.1093/intimm/13.3.273
Reichelt J, Haase I (2010) Establishment of spontaneously immortalized keratinocyte lines from wild-type and mutant mice. Methods Mol Biol 585:59–69. https://doi.org/10.1007/978-1-60761-380-0_5
Rötzer V, Hartlieb E, Winkler J, Walter E, Schlipp A, Sardy M, Spindler V, Waschke J (2016) Desmoglein 3-dependent signaling regulates keratinocyte migration and wound healing. J Invest Dermatol 136(1):301–310. https://doi.org/10.1038/JID.2015.380
Schaeffer HJ, Weber MJ (1999) Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol 19(4):2435–2444. https://doi.org/10.1128/MCB.19.4.2435
Shirakata Y, Amagai M, Hanakawa Y, Nishikawa T, Hashimoto K (1998) Lack of mucosal involvement in pemphigus foliaceus may be due to low expression of desmoglein 1. J Invest Dermatol 110(1):76–78. https://doi.org/10.1046/j.1523-1747.1998.00085.x
Siljamäki E, Raiko L, Toriseva M, Nissinen L, Näreoja T, Peltonen J, Kähäri VM, Peltonen S (2014) p38delta mitogen-activated protein kinase regulates the expression of tight junction protein ZO-1 in differentiating human epidermal keratinocytes. Arch Dermatol Res 306(2):131–141. https://doi.org/10.1007/s00403-013-1391-0
Stanley JR, Amagai M (2006) Pemphigus, bullous impetigo, and the staphylococcal scalded-skin syndrome. N Engl J Med 355(17):1800–1810. https://doi.org/10.1056/NEJMra061111
Vollmers A, Wallace L, Fullard N, Höher T, Alexander MD, Reichelt J (2012) Two- and three-dimensional culture of keratinocyte stem and precursor cells derived from primary murine epidermal cultures. Stem Cell Rev Rep 8(2):402–413. https://doi.org/10.1007/s12015-011-9314-y
Waschke J, Spindler V (2014) Desmosomes and extradesmosomal adhesive signaling contacts in pemphigus. Med Res Rev 34(6):1127–1145. https://doi.org/10.1002/med.21310
Watabe M, Nagafuchi A, Tsukita S, Takeichi M (1994) Induction of polarized cell-cell association and retardation of growth by activation of the E-cadherin-catenin adhesion system in a dispersed carcinoma line. J Cell Biol 127(1):247–256. https://doi.org/10.1083/jcb.127.1.247
Zhu AJ, Watt FM (1996) Expression of a dominant negative cadherin mutant inhibits proliferation and stimulates terminal differentiation of human epidermal keratinocytes. J Cell Sci 109(13):3013–3023. https://doi.org/10.1242/jcs.109.13.3013
Acknowledgements
The authors would like to thank Editage for English proofreading.
Funding
This study was supported in part by a Grant-in-Aid for Scientific Research (C) (No. 22K09921) from the Ministry of Education, Culture, Sports, Science, and Technology in Japan.
Author information
Authors and Affiliations
Contributions
T.In. conceived and planned the experiments. T.In. and T.Is. established DSG3 KO cells. S.O. carried out all experiments with help from T.O., Y.I., T.M., and T.In., and T.In. contributed to the interpretation of the results. Y.I. analyzed data of dispase-based dissociation assay. All authors read and approved the final manuscript. T.In. wrote the manuscript with input from all authors and supervised the project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Negative controls for immunofluorescence. Confluent WT (a, b) and KO (c, d) cells were cultured for one day (a, c) or three days (b, d) in the presence of 1.2 mM calcium. Confluent KO cells were cultured for 6 h in a high-calcium medium with a vehicle (e), 20 μM BIRB 796 (f), or 20 μM SP600125 (g). Confluent WT cells were cultured for 6 h in a high-calcium medium with a vehicle (h), 100 nM anisomycin (i), 100 nM anisomycin plus 20 μM BIRB 796 (j), or 100 nM anisomycin plus 20 μM SP600125 (k). They were incubated with a mixture of anti-mouse and anti-rabbit immunoglobulin conjugated with Alexa 488 or Alexa 568, omitting the primary antibodies. Nuclei were stained with DAPI, and merged images are shown. No specific immunofluorescence was observed. Scale bar: 20 μm. Supplementary file1 (TIF 8886 KB)
Supplementary Fig. 2
The localization of DSG1 decreases in cell–cell contacts of KO cells cultured for one and three days. Confluent KO cells (clone 207-46) were cultured for one (a–f) and three (g–l) days in the presence of 1.2 mM calcium. Cells were double-stained with antibodies against either DSG1 (a, g, green) and E-cadherin (Ecad) (b, h, red) or DSG3 (d, j, green) and DSG2 (e, k, red). Merged images are shown in c, f, i, and l. Nuclei were stained with DAPI (c, f, i, l, blue). After one day of culture, some cell clusters growing on top of the KO cell monolayer (hereafter referred to as surface cells) showed weak to moderate DSG1 staining in the cytoplasm and cell–cell contacts (asterisks in a), while cells that adhered to the glass slides (hereafter referred to as basal cells) showed weak DSG1 staining (a). Arrowheads indicate cell–cell contacts between basal cells that had faint staining for DSG1 (a) but intense staining for Ecad (b). Two single cells (arrows in a) on top of the KO cell monolayer that were not in contact with other surface cells showed very strong DSG1 staining in the cytoplasm (a). DSG3 staining was not detected in KO cells (d). DSG2 staining in cell–cell contacts (e) was heterogeneous and weak. After three days of culture, the area of surface cell clusters increased, and surface cells showed weak to strong DSG1 staining in the cytoplasm (g). Occasionally, some gaps (asterisks in g–i) were observed between basal cells. These gaps were visible thorough three surface cells with weak to moderate cytoplasmic DSG1 staining (outlined by arrowheads in g). DSG3 staining was not detected in KO cells (j). Compared to one day of culture, DSG2 staining in cell–cell contacts was slightly stronger (k). Scale bar: 20 μm. Supplementary file2 (TIF 15810 KB)
Supplementary Fig. 3
BIRB 796 and SP600125 inhibited the phosphorylation (activation) of p38 MAPK and JNK, respectively, in KO cells treated with anisomycin. Confluent KO cells (clone 207-4) were cultured for 0.5 h in a high-calcium medium with a vehicle (control), 100 nM anisomycin (AM), AM plus 20 μM BIRB 796 (BIRB), or AM plus 20 μM SP600125 (SP). Total cell lysates were separated by SDS-PAGE, followed by western blotting with antibodies to phospho-p38 MAPK (P-p38), p38 MAPK (p38), phospho-JNK (P-JNK), JNK and actin. P-JNK was not detected in the control KO cells. AM increased the immunoreactivity of both P-p38 and P-JNK compared with the control. In AM-treated KO cells, BIRB 796 or SP600125 specifically inhibited the phosphorylation of p38 or JNK, respectively. Supplementary file3 (TIF 677 KB)
Supplementary Fig. 4
Linear localization of DSG1 to cell–cell contacts is promoted by JNK inhibition in KO cells. Confluent KO cells (clone 207-46) were cultured for 6 h in a high-calcium medium with a vehicle (a), 20 μM BIRB 796 (b), or 20 μM SP600125 (c). Cells were stained with an antibody against DSG1. In control (a), negative, faint, or zipper-like (arrows) immunoreactivity for DSG1 was detected in cell–cell contacts. BIRB 796 treatment (b) promoted zipper-like (arrows) and linear (arrowheads) localization of DSG1 to cell–cell contacts. SP600125 treatment (c) promoted the linear (arrowheads) localization of DSG1 to cell–cell contacts. Scale bar: 10 μm. Supplementary file4 (TIF 3947 KB)
Supplementary Fig. 5
The inhibition of JNK restores cell–cell adhesion in KO cells. Confluent WT cells were cultured for 24 h in the presence of calcium. Confluent KO cells (clone 207-46) were cultured for 24 h in the presence of calcium or calcium plus 20 μM SP600125 (SP). Cell monolayers were detached from 24-well plates by dispase, transferred to 1.5 ml tubes, vortexed, and transferred to 24-well plates. Representative images of cell monolayers subjected to dispase-based dissociation assays are shown. The number of cell monolayer fragments was counted (n = 4). Compared to the control, the number of KO cell monolayer fragments was reduced by 78.1% with SP treatment. Statistical significance was set as *p < 0.05, **p < 0.01, and ***p < 0.001. ns: not significant. Supplementary file5 (TIF 20591 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ogawa, S., Ishii, T., Otani, T. et al. JNK inhibition enhances cell–cell adhesion impaired by desmoglein 3 gene disruption in keratinocytes. Histochem Cell Biol 161, 345–357 (2024). https://doi.org/10.1007/s00418-023-02264-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-023-02264-8