Abstract
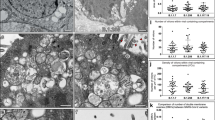
Despite being extensively studied because of the current coronavirus disease 2019 (COVID-19) pandemic, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) interactions with mammalian cells are still poorly understood. Furthermore, little is known about this coronavirus cycle within the host cells, particularly the steps that lead to viral egress. This study aimed to shed light on the morphological features of SARS-CoV-2 egress by utilizing transmission and high-resolution scanning electron microscopy, along with serial electron tomography, to describe the route of nascent virions towards the extracellular medium. Electron microscopy revealed that the clusters of viruses in the paracellular space did not seem to result from collective virus release. Instead, virus accumulation was observed on incurved areas of the cell surface, with egress primarily occurring through individual vesicles. Additionally, our findings showed that the emission of long membrane projections, which could facilitate virus surfing in Vero cells infected with SARS-CoV-2, was also observed in non-infected cultures, suggesting that these are constitutive events in this cell lineage.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
Alsaadi EJA, Jones IM (2019) Membrane binding proteins of coronaviruses. Future Virol 14(4):275–286
Barreto-Vieira DF, da Silva MAN, Garcia CC et al (2021) Morphology and morphogenesis of SARS-CoV-2 in Vero-E6 cells. Mem Inst Oswaldo Cruz 116:e200443
Barreto-Vieira DF, da Silva MAN, de Almeida ALT et al (2022) SARS-CoV-2: ultrastructural characterization of morphogenesis in an in vitro system. Viruses 14(2):201
Baselga M, Moreo E, Uranga-Murillo I, Arias M, Junquera C (2023) Ultrastructural analysis and three-dimensional reconstruction of cellular structures involved in SARS-CoV-2 spread. Histochem Cell Biol 159(1):47–60
Blanchard E, Roingeard P et al (2015) Virus-induced double-membrane vesicles. Cell Microbiol 17:45–50
Caldas LA, Carneiro FA, Higa LM et al (2020) Ultrastructural analysis of SARS-CoV-2 interactions with the host cell via high resolution scanning electron microscopy. Sci Rep 10(1):16099
Caldas LA, Carneiro FA, Monteiro FL et al (2021) Intracellular host cell membrane remodelling induced by SARS-CoV-2 infection in vitro. Biol Cell 113(6):281–293
Chen D, Zheng Q, Sun L et al (2021) ORF3a of SARS-CoV-2 promotes lysosomal exocytosis-mediated viral egress. Dev Cell S1534–5807(21):00807–00808
Ducatelle R, Hoorens J (1984) Significance of lysosomes in the morphogenesis of coronaviruses. Arch Virol 79:1–12
Eymieux S, Uzbekov R, Rouillé Y et al (2021a) Secretory vesicles are the principal means of SARS-CoV-2 egress. Cells 10(8):2047
Eymieux S, Rouillé Y, Terrier O et al (2021b) Ultrastructural modifications induced by SARS-CoV-2 in Vero cells: a kinetic analysis of viral factory formation, viral particle morphogenesis and virion release. Cell Mol Life Sci 78(7):3565–3576
Ghosh S, Dellibovi-Ragheb TA, Kerviel A et al (2020) β-Coronaviruses use lysosomes for egress instead of the biosynthetic secretory pathway. Cell 183(6):1520-1535.e14
Kim JM, Chung YS, Jo HJ et al (2020) Identification of coronavirus isolated from a patient in Korea with COVID-19. Osong Public Health Res Perspect 11(1):3–7
Klein S, Cortese M, Winter SL et al (2019) SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography. Nat Commun 11(1):5885
Kloc M, Uosef A, Wosik J, Kubiak JZ, Ghobrial RM (2022) Virus interactions with the actin cytoskeleton-what we know and do not know about SARS-CoV-2. Arch Virol 167(3):737–749
Kopek BG, Perkins G, Miller DJ, Ellisman MH, Ahlquist P (2007) Three-dimensional analysis of a viral RNA replication complex reveals a virus-induced mini-organelle. PLoS Biol 5(9):e220
Lehmann MJ, Sherer NM, Marks CB et al (2005) Actin and myosin driven movement of viruses along filopodia precedes their entry into cells. J Cell Biol 170:317–325. https://doi.org/10.1083/jcb.200503059
Lu R, Zhao X, Li J et al (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395(10224):565–574
Mendonça L, Howe A, Gilchrist JB et al (2021) Correlative multi-scale cryo-imaging unveils SARS-CoV-2 assembly and egress. Nat Commun 12(1):4629
Park WB, Kwon NJ, Choi SJ et al (2020) Virus isolation from the first patient with SARS-CoV-2 in Korea. J Korean Med Sci 35(7):e84
Pepe A, Pietropaoli S, Vos M et al (2022) Tunneling nanotubes provide a route for SARS-CoV-2 spreading. Sci Adv 8(29):eabo171
Perlman S, Netland J (2009) Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol 7:439–450
Pinto AL, Rai RK, Brown JC et al (2022) Ultrastructural insight into SARS-CoV-2 entry and budding in human airway epithelium. Nat Commun 13(1):1609
Pu J, Guardia CM, Keren-Kaplan T, Bonifacino JS (2016) Mechanisms and functions of lysosome positioning. J Cell Sci 129:4329–4339
Qian Z, Travanty EA, Oko L et al (2013) Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am J Respir Cell Mol Biol 48:742–748
Qinfen Z, Jinming C, Xiaojun H et al (2004) The life cycle of SARS coronavirus in Vero E6 cells. J Med Virol 73(3):332–337
Saraste J, Enyioko M, Dale H et al (2022) Evidence for the role of Rab11-positive recycling endosomes as intermediates in coronavirus egress from epithelial cells. Histochem Cell Biol 158(3):241–251
Scherer KM, Mascheroni L, Carnell GW et al (2022) SARS-CoV-2 nucleocapsid protein adheres to replication organelles before viral assembly at the Golgi/ERGIC and lysosome-mediated egress. Sci Adv 8(1):eabl4895
Snijder EJ, Limpens RWAL, de Wilde AH et al (2020) A unifying structural and functional model of the coronavirus replication organelle: tracking down RNA synthesis. PLoS Biol 18:1–25
Sylwester A, Murphy S, Shutt D et al (1997) HIV-induced T cell syncytia are self-perpetuating and the primary cause of T cell death in culture. J Immunol 158(8):3996–4007
Wolff G, Limpens R, Zevenhoven-Dobbe JC (2020a) A molecular pore spans the double membrane of the coronavirus replication organelle. Science 369(6509):1395–1398
Wolff G, Melia CE, Snijder EJ, Bárcena M (2020b) Double-membrane vesicles as platforms for viral replication. Trends Microbiol 28(12):1022–1033
Zhang J, Lan Y, Sanyal S (2020) Membrane heist: Coronavirus host membrane remodeling during replication. Biochimie 179:229–236
Zhou X, Cong Y, Veenendaal T et al (2017) Ultrastructural characterization of membrane rearrangements induced by porcine epidemic diarrhea virus infection. Viruses 9(9):251
Acknowledgements
We thank Adelia Mara Belém Lima and Lorian Cobra Straker for support in the electron microscope at CENABIO facilities; Willian Ferreira da Silva and Bráulio Soares Archanjo for support in the Orion Microscope at INMETRO facilities. We also thank Edison Luiz Durigon (USP), Ester Sabino (IMT-SP), Fernando Spike (FEEVALE-SC) and João Renato Rebello Pinho (HIAE).
Funding
Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro–FAPERJ, grant No. E-26/010.000978/2019; Financiadora de Estudos e Projetos–FINEP; Conselho Nacional de Desenvolvimento Científico e Tecnológico–CNPq.
Author information
Authors and Affiliations
Contributions
I.A.C. and L.J.C. conducted the Isolate preparations, virus growth, and infections; L.A.C. and F.A.C. performed the sample preparations and microscopy analysis; I.A. and K.M. performed the FIB and tomographic image alignment and reconstruction; L.A.C., F.A.C., L.J.C., K.M. and W.S. performed the analysis of the results; W.S. and A.T. contributed to the initial conception and design of this work; L.A.C. and F.A.C. wrote the first draft of the manuscript. All the authors were involved in reviewing and editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Supplementary file1 Tomogram showing viruses held in plasma membrane invagination. Two slices of 200 nm were used (MP4 9411 KB)
Supplementary file2 Infected Vero cells processed by FIB. Purple, plasma membrane; yellow, virus; light blue, viral factory; blue cyan, LVCV; dark blue, nucleus; brown, mitochondria (MP4 71874 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Caldas, L.A., Carneiro, F.A., Augusto, I. et al. SARS-CoV-2 egress from Vero cells: a morphological approach. Histochem Cell Biol 161, 59–67 (2024). https://doi.org/10.1007/s00418-023-02239-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-023-02239-9