Abstract
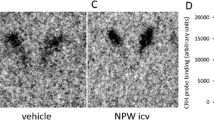
Previous immunocytochemical studies have shown the presence of motilin-immunoreactive neurons in specific brain areas of rats and autoradiographic studies in rabbits demonstrated motilin-binding sites in the central nervous system as well. Therefore, the aim of this study was to determine the anatomical localisation and neurochemical features of neurons activated by central administration of motilin (Mo) in rats. One week after cannulation, an intracerebroventricular injection of Mo (ICV, 3 μg/6 μl 0.9% saline) was given. For comparative purposes, a group of animals received an intravenous injection of motilin (IV, 9 μg/300 μl 0.9% saline) or an equal volume of saline. Neuronal excitation was assessed by c-Fos immunocytochemistry and combined with immunostaining for neurotransmitter markers. In contrast to the IV motilin-treated animals, the ICV motilin-treated animals displayed a significant increase in c-Fos expression in the supraoptic nuclei (SO) and paraventricular nuclei of the hypothalamus (PVH). At the level of the dorsomedial, ventromedial and lateral hypothalamic nuclei, ICV administration of motilin did not induce changes in c-Fos expression. In addition, the cerebellum did not show c-Fos expression after ICV motilin administration either. These findings might suggest distinct pathways and actions of centrally released and systemic motilin, but, particularly in rodents, do not rule out the possibility that the effects seen in the SO and PVH after ICV application are aspecific in nature. At present, we cannot exclude the fact that the results observed with motilin in rodents are due to cross-interaction with other related (e.g. ghrelin) or not yet identified receptors.



Similar content being viewed by others
References
Abbott CR, Kennedy AR, Wren AM, Rossi M, Murphy KG, Seal LJ, Todd JF, Ghatei MA, Small CJ, Bloom SR (2003) Identification of hypothalamic nuclei involved in the orexigenic effect of melanin-concentrating hormone. Endocrinology 144:3943–3949
Aerssens J, Depoortere I, Thielemans L, Mitselos A, Coulie B, Peeters TL (2004) The rat lacks functional genes for motilin and for the motilin receptor. Abstract XII European Symposium on Neurogastroenterology and Motility, p 100
Asakawa A, Inui A, Momose K, Ueno N, Fujino MA, Kasuga M (1998) Motilin increases food intake in mice. Peptides 19:987–990
Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M (2001) Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 120:337–345
Brown JC, Cook MA, Dryburgh JR (1973) Motilin, a gastric motor activity stimulating polypeptide: the complete amino acid sequence. Can J Biochem 51:533–537
Dass NB, Hill J, Muir A, Testa T, Wise A, Sanger GJ (2003) The rabbit motilin receptor: molecular characterisation and pharmacology. Br J Pharmacol 140:948–954
Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M (1999) Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA 96:748–753
Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M (2002) The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123:1120–1128
Depoortere I (2001) Motilin and motilin receptors: characterization and functional significance. Verh K Acad Geneeskd Belg 63:511–529
Depoortere I, Peeters TL (1997) Demonstration and characterization of motilin-binding sites in the rabbit cerebellum. Am J Physiol Gastrointest L 35:G994-G999
Depoortere I, Van Assche G, Peeters TL (1997) Distribution and subcellular localization of motilin binding sites in the rabbit brain. Brain Res 777:103–109
Feighner SD, Tan CP, McKee KK, Palyha OC, Hreniuk DL, Pong SS, Austin CP, Figueroa D, MacNeil D, Cascieri MA, Nargund R, Bakshi R, Abramovitz M, Stocco R, Karman S, O’Neill G, Van der Ploeg LH, Evans J, Patchett AA, Smith RG, Howard AD (1999) Receptor for motilin identified in the human gastrointestinal system. Science 284:2184–2188
Garthwaite TL (1985) Peripheral motilin administration stimulates feeding in fasted rats. Peptides 6:41–44
Guan Y, Tang M, Jiang Z, Peeters TL (2003) Excitatory effects of motilin in the hippocampus on gastric motility in rats. Brain Res 984:33–41
Herdegen T, Kovary K, Buhl A, Bravo R, Zimmermann M, Gass P (1995) Basal expression of the inducible transcription factors c-jun, junB, junD, c-fos, fosB, and krox-24 in the adult rat brain. J Comp Neurol 354:39–56
Huang Z, Depoortere I, De Clercq P, Peeters TL (1999) Sequence and characterization of cDNA encoding the motilin precursor from chicken, dog, cow and horse. Evidence of mosaic evolution in prepromotilin. Gene 240:217–226
Ida T, Nakahara K, Katayama T, Murakami N, Nakazato M (1999) Effect of lateral cerebroventricular injection of the appetite-stimulating neuropeptide, orexin and neuropeptide Y, on the various behavioral activities of rats. Brain Res 821:526–529
Itoh Z (1997) Motilin and clinical application. Peptides 18:593–608
Jacobowitz DM, O’Donohue TL, Chey WY, Chang T-M (1981) Mapping of motilin-immunoreactive neurons of the rat brain. Peptides 2:479–487
Kyrkouli SE, Stanley BG, Seirafi RD, Leibowitz SF (1990) Stimulation of feeding by galanin: anatomical localization and behavioral specificity of this peptide’s effects in the brain. Peptides 11:995–1001
Lange W, Unger J, Pitzl H, Weindl A (1986) Is motilin a cerebellar peptide in the rat? A radioimmunological, chromatographic and immunohistochemical study. Anat Embryol 173:371–376
Lu S, Guan JL, Wang QP, Uehara K, Yamada S, Goto N, Date Y, Nakazato M, Kojima M, Kangawa K, Shioda S (2002) Immunocytochemical observation of ghrelin-containing neurons in the rat arcuate nucleus. Neurosci Lett 321:157–160
Makarenko IG, Meguid MM, Gatto L, Chen C, Ugrumov MV (2003) Decreased NPY innervation of the hypothalamic nuclei in rats with cancer anorexia. Brain Res 961:100–108
Melis MR, Mascia MS, Succu S, Torsello A, Muller EE, Deghenghi R, Argiolas A (2002) Ghrelin injected into the paraventricular nucleus of the hypothalamus of male rats induces feeding but not penile erection. Neurosci Lett 329:339–343
Momose K, Inui A, Asakawa A, Ueno N, Nakajima M, Kasuga M (1998) Anxiolytic effect of motilin and reversal with GM-109, a motilin antagonist, in mice. Peptides 19:1739–1742
Morgan JI, Curran T (1988) Calcium as a modulator of the immediate-early gene cascade in neurons. Cell 9:303–311
Morley JE, Levine AS, Yim GK, Lowy MT (1983) Opioid modulation of appetite. Neurosci Biobehav Rev 7:281–305
Nagase H, Nakajima A, Sekihara H, York DA, Bray GA (2002) Regulation of feeding behavior, gastric emptying, and sympathetic nerve activity to interscapular brown adipose tissue by galanin and enterostatin: the involvement of vagal-central nervous system interactions. J Gastroenterol 37:118–127
Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S (2001) A role for ghrelin in the central regulation of feeding. Nature 409:194–198
Nilaver G, Defendini R, Zimmerman EA, Beinfield MC, O’Donohue TL (1982) Motilin in the Purkinje cell of the cerebellum. Nature 295:597–598
O’Donohue TL, Beinfeld MC, Chey WY, Chang T-M, Nilaver G, Zimmerman EA, Yajima H, Adachi H, Poth M, McDevitt RP, Jacobowitz DM (1981) Identification, characterization and distribution of motilin immunoreactivity in the rat central nervous system. Peptides 2:467–477
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic, New York
Peeters TL (1993) Erythromycin and other macrolides as prokinetic agents. Gastroenterology 105:1886–1899
Rosenfeld DJ, Garthwaite TL (1987) Central administration of motilin stimulates feeding in rats. Physiol Behav 39:753–756
Rowland NE, Farnbauch LJ, Robertson KL (2003) Brain muscarinic receptor subtypes mediating water intake and Fos following cerebroventricular administration of bethanecol in rats. Psychopharmacology 167:174–179
Schwartz MW, Woods SC, Porte JrD, Seeley RJ, Baskin DG (2000) Central nervous system control of food intake. Nature 404:661–671
Stanley BG, Daniel DR, Chin AS, Leibowitz SF (1985) Paraventricular nucleus injections of peptide YY and neuropeptide Y preferentially enhance carbohydrate ingestion. Peptides 6:1205–1211
Stanley BG, Ha, LH, Spears LC, Dee MG (1993) Lateral hypothalamic injections of glutamate, kainic acid, D,L-alpha-amino-3-hydroxy-5-methyl-isoxazole propionic acid or N-methyl-D-aspartic acid rapidly elicit intense transient eating in rats. Brain Res 613:88–95
Tempel DL, Leibowitz KJ, Leibowitz SF (1988) Effects of PVN galanin on macronutrient selection. Peptides 9:309–314
Thielemans L, Depoortere I, Van Assche G, Bender E, Peeters TL (2001) Demonstration of a functional motilin receptor in TE671 cells from human cerebellum. Brain Res 895:119–128
Toshinai K, Date Y, Murakami N, Shimada M, Mondal MS, Shimbara T, Guan JL, Wang OP, Funahashi H, Sakurai T, Shioda S, Matsukura S, Kangawa K, Nakazato M (2003) Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology 144:1506–1512
Tsukamura H, Tsukahara S, Maekawa F, Moriyama R, Reyes BA, Sakai T, Niwa Y, Foster DL (2000) Peripheral or central administration of motilin suppresses LH release in female rats: a novel role for motilin. J Neuroendocrinol 12:403–408
Van der Lely AJ, Tschöp M, Heiman ML, Ghigo E (2004) Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev 25:426–457
Wang L, Saint-Pierre DH, Taché Y (2002) Peripheral ghrelin selectively increases Fos expression in neuropeptide Y-synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett 325:47–51
Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DGA, Ghatei MA, Bloom SR (2000) The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141:4325–4328
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, M., Tang, M., Adriaensen, D. et al. Central, but not peripheral application of motilin increases c-Fos expression in hypothalamic nuclei in the rat brain. Histochem Cell Biol 123, 139–145 (2005). https://doi.org/10.1007/s00418-005-0763-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-005-0763-8