Abstract
Purpose
To assess 6-month outcomes of switching from aflibercept to faricimab in eyes with refractory neovascular age-related macular degeneration (nAMD) previously requiring monthly injections.
Methods
This multicenter retrospective study examined nAMD eyes receiving monthly aflibercept injections switched to faricimab administered monthly up to 4 injections followed by injections at a minimum of 2-month intervals as per drug labeling. Data regarding age, sex, number of previous injections, treatment intervals, and best-corrected visual acuity (BCVA) were collected. Central retinal thickness (CRT), subfoveal choroidal thickness (SFCT), and maximal pigment epithelial detachment (PED) height were measured by optical coherence tomography.
Results
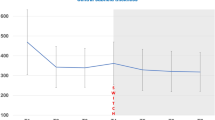
The study included 130 eyes of 124 patients. At 6 months, 53 eyes (40.8%) continued on faricimab treatment (Group 1), while 77 eyes (59.2%) discontinued faricimab for various reasons (Group 2) the most common being worse exudation. There were no significant differences between the two groups at baseline. In Group 1, CRT and SFCT significantly decreased at 1 month (P = 0.013 and 0.008), although statistical significance was lost at 6 months (P = 0.689 and 0.052). BCVA and maximal PED height showed no significant changes; however, mean treatment intervals were extended from 4.4 ± 0.5 weeks at baseline to 8.7 ± 1.7 weeks at 6 months (P < 0.001) in Group 1. No clear predictors of response were identified.
Conclusion
Switching from aflibercept to faricimab allowed for extension of treatment intervals from monthly to bimonthly in roughly 40% of eyes, suggesting that faricimab may be considered in refractory nAMD cases.


Similar content being viewed by others
References
Wykoff CC, Ou WC, Brown DM, Croft DE, Wang R, Payne JF, Clark LW, Abdelfattah N, Sadda SR, the TREX-AMD Study Group (2017) Randomized trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration 2-year results of the trex-amd study. Ophthalmol Retina 1:314–321. https://doi.org/10.1016/j.oret.2016.12.004
Rosenberg D, Deonarain DM, Gould J, Sothivannan A, Phillips MR, Sarohia GS, Sivaprasad S, Wykoff CC, Cheung CMG, Sarraf D, Bakri SJ, Chaudhary V (2023) Efficacy, safety, and treatment burden of treat-and-extend versus alternative anti-VEGF regimens for nAMD: a systematic review and meta-analysis. Eye (Lond) 37:6–16. https://doi.org/10.1038/s41433-022-02020-7
Yamamoto A, Okada AA, Nakayama M, Yoshida Y, Kobayashi H (2017) One-year outcomes of a treat-and-extend regimen of aflibercept for exudative age-related macular degeneration. Ophthalmologica 237:139–144. https://doi.org/10.1159/000458538
Tsunekawa Y, Kataoka K, Asai K, Ito Y, Terasaki H (2021) Four-year outcome of aflibercept administration using a treat-and-extend regimen in eyes with recurrent neovascular age-related macular degeneration. Jpn J Ophthalmol 65:69–76. https://doi.org/10.1007/s10384-020-00783-8
Mitchell P, Holz FG, Hykin P, Midena E, Souied E, Allmeier H, Lambrou G, Schmelter T, Wolf S, the ARIES study investigators (2021) Efficacy and safety of intravireal aflibercept using a treat-and-extend regimen for neovascular age-related macular degeneration: the ARIES study: a randomized clinical trial. Retina 41: 1911-1920. https://doi.org/10.1097/IAE.0000000000003128
Gillies MC, Hunyor AP, Arnold JJ, Guymer RH, Wolf S, Ng P, Pecheur FL, McAllister IL (2019) Effect of ranibizumab and aflibercept on best-corrected visual acuity in treat-and-extend for neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmol 137:372–379. https://doi.org/10.1001/jamaophthalmol.2018.6776
Gillies MC, Hunyor AP, Arnold JJ, Guymer RH, Wolf S, Pecheur FL, Munk MR, McAllister IL (2020) Macular atrophy in neovascular age-related macular degeneration: a randomized clinical trial comparing ranibizumab and aflibercept (RIVAL study). Ophthalmology 127:198–210. https://doi.org/10.1016/j.ophtha.2019.08.023
Maruko I, Ogasawara M, Yamamoto A, Itagaki K, Hasegawa T, Arakawa H, Nakayama M, Koizumi H, Okada AA, Sekiryu T, Iida T (2020) Two-year outcomes of treat-and-extend intravitreal aflibercept for exudative age-related macular degeneration: a prospective study. Ophthalmol Retina 4:767–776. https://doi.org/10.1016/j.oret.2020.03.010
Heier JS, Singh RP, Wykoff CC, Csaky KG, Lai TYY, Loewenstein A, Schlottmann PG, Paris LP, Westenskow PD, Quezada-Ruiz C (2021) The angiopoietin/tie pathway in retinal vascular diseases: a review. Retina 41:1–19. https://doi.org/10.1097/IAE.0000000000003003
Oshima Y, Deering T, Oshima S, Nambu H, Reddy PS, Kaleko M, Connelly S, Hackett SF, Campochiaro PA (2004) Angiopoietin-2 enhances retinal vessel sensitivity to vascular endothelial growth factor. J Cell Physiol 199:412–417. https://doi.org/10.1002/jcp.10442
Regula JT, Lundh von Leithner P, Foxton R, Barathi VA, Cheung CM, Bo Tun SB, Wey YS, Iwata D, Dostalek M, Moelleken J, Stubenrauch KG, Nogoceke E, Widmer G, Strassburger P, Koss MJ, Klein C, Shima DT, Hartmann G (2016) Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol Med 8:1265–1288. https://doi.org/10.15252/emmm.201505889
Heier JS, Khanani AM, Ruiz CQ, Karen Basu PJF, Brittain C, Figueroa MS, Lin H, Holz FG, Patel V, Lai TYY, Silverman D, Regillo C, Swaminathan B, Viola F, Cheung CMG, Wong TY, the TENAYA and LUCERNE Investigators (2022) Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet 399:729–740. https://doi.org/10.1016/s0140-6736(22)00010-1
de Oliveira Dias JR, Rodrigues EB, Maia M, Magalhaes O Jr, Penha FM, Farah ME (2011) Cytokines in neovascular age-related macular degeneration: fundamentals of targeted combination therapy. Br J Ophthalmol 95:1631–1637. https://doi.org/10.1136/bjo.2010.186361
Yang S, Zhao J, Sun X (2016) Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: a comprehensive review. Drug Des Devel Ther 10:1857–1867. https://doi.org/10.2147/DDDT.S97653
Ferrara N, Damico L, Shams N, Lowman H, Kim R (2006) Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 26:859–870. https://doi.org/10.1097/01.iae.0000242842.14624.e7
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Group MS (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355:1419–1431. https://doi.org/10.1056/NEJMoa054481
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S, ANCHOR Study Group (2006) Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 355:1432–1444. https://doi.org/10.1056/NEJMoa062655
Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, Ioffe E, Huang T, Radziejewski C, Bailey K, Fandl JP, Daly T, Wiegand SJ, Yancopoulos GD, Rudge JS (2002) VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA 99:11393–11398. https://doi.org/10.1073/pnas.172398299
Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, Pyles EA, Yancopoulos GD, Stahl N, Wiegand SJ (2012) Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 15:171–185. https://doi.org/10.1007/s10456-011-9249-6
Cho H, Shah CP, Weber M, Heier JS (2013) Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol 97:1032–1035. https://doi.org/10.1136/bjophthalmol-2013-303344
Yonekawa Y, Andreoli C, Miller JB, Loewenstein JI, Sobrin L, Eliott D, Vavvas DG, Miller JW, Kim IK (2013) Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am J Ophthalmol 156:29–35. https://doi.org/10.1016/j.ajo.2013.03.030
Holz FG, Dugel PU, Weissgerber G, Hamilton R, Silva R, Bandello F, Larsen M, Weichselberger A, Wenzel A, Schmidt A, Escher D, Sararols L, Souied E (2016) Single-chain antibody fragment VEGF inhibitor RTH258 for neovascular age-related macular degeneration a randomized controlled study. Ophthalmology 123:1080–1089. https://doi.org/10.1016/j.ophtha.2015.12.030
Ota H, Takeuchi J, Nakano Y, Horiguchi E, Taki Y, Ito Y, Terasaki H, Nishiguchi KM, Kataoka K (2022) Switching from aflibercept to brolucizumab for the treatment of refractory neovascular age-related macular degeneration. Jpn J Ophthalmol. https://doi.org/10.1007/s10384-022-00908-1
Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, Gomes AV, Warburton J, Weichselberger A, Holz FG, the HAWK and HARRIER Study Investigators (2020) HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 127:72–84. https://doi.org/10.1016/j.ophtha.2019.04.017
Maruko I, Okada AA, Iida T, Hasegawa T, Izumi T, Kawai M, Maruko R, Nakayama M, Yamamoto A, Koizumi H, Tamashiro T, Terao N, Wakugawa S, Mori R, Onoe H, Tanaka K, Wakatsuki Y, Itagaki K, Kasai A et al (2021) Brolucizumab-related intraocular inflammation in Japanese patients with age-related macular degeneration: a short-term multicenter study. Graefes Arch Clin Exp Ophthalmol. https://doi.org/10.1007/s00417-021-05136-w
Matsumoto H, Hoshino J, Mukai R, Nakamura K, Akiyama H (2021) Short-term outcomes of intravitreal brolucizumab for treatment-naive neovascular age-related macular degeneration with type 1 choroidal neovascularization including polypoidal choroidal vasculopathy. Sci Rep 11:6759. https://doi.org/10.1038/s41598-021-86014-7
Acknowledgements
Japan AMD Research Consortium (JARC): Makiko Nakayama, MD, PhD, Akiko Yamamoto, MD, PhD, Keiko Kataoka, MD, PhD, and Annabelle A. Okada, MD, PhD (Department of Ophthalmology, Kyorin University School of Medicine, Japan); Kanako Itagaki, MD, Masashi Ogasawara, MD, Junichiro Honjyo, MD, Ryo Mukai, MD, PhD, and Tetsuju Sekiryu, MD, PhD (Department of Ophthalmology, Fukushima Medical University, Japan); Nozomu Hashiya, MD, Ichiro Maruko, MD, PhD, Taiji Hasegawa, MD, PhD, Moeko Kawai, MD, PhD, Ruka Maruko, MD, PhD, and Tomohiro Iida, MD, PhD (Department of Ophthalmology, Tokyo Women’s Medical University, Japan); Sorako Wakugawa, MD, Yasunori Miyara, MD, PhD, Nobuhiro Terao, MD, PhD, and Hideki Koizumi, MD, PhD (Department of Ophthalmology, University of the Ryukyus, Japan); Koji Tanaka, MD, PhD, Hajime Onoe, MD, Yu Wakatsuki, MD, PhD, and Ryusaburo Mori, MD, PhD (Department of Ophthalmology, Nihon University School of Medicine, Japan)
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study’s conception and design. Data collection and analysis were performed by Keiko Kataoka, Kanako Itagaki, Nozumu Hashiya, Sorako Wakugawa, and Koji Tanaka. The first draft of the manuscript was written by Keiko Kataoka and revised by Annabelle A. Okada. All authors read the manuscript, provided comments, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This retrospective study was conducted in accordance with the guidelines of the Helsinki Declaration. The Institutional Review Boards of Kyorin University, Fukushima Medical University, Tokyo Women’s Medical University, University of the Ryukyus, and Nihon University approved the study.
Competing interests
Iida T. received grants from Nidek, Topcon, Santen, Novartis Pharma, Senju Pharmaceutical, Japan Alcon, HOYA, and AMO; consultant fee from Bayer Pharmaceuticals, Novartis Pharma, Chugai Pharmaceutical, Japan Boehringer Ingelheim, and Janssen Pharma; and received lecturing and travel fees from Bayer Pharmaceuticals, Novartis Pharma, Japan Alcon, Santen, Senju Pharmaceutical, Topcon, Chugai Pharmaceutical, Canon, Nidek, Otsuka Pharmaceutical, Nikon, and Kyowa Kirin. He holds a patent. Maruko I. received lecturing and travel fees from Bayer Pharmaceuticals, Novartis Pharma, Japan Alcon, Santen, Senju Pharmaceutical, Topcon, Chugai Pharmaceutical, Canon, Nidek, and Nikon. He holds a patent. Hasegawa T. received lecturing and travel fees from Bayer Pharmaceuticals, Novartis Pharma, Alcon Pharma, Santen, Kowa, Senju Pharmaceutical, R.E. Medical, Nikon Health Care Japan, JFC Sales Plan, Otsuka Pharmaceutical, and Japan Boehringer Ingelheim. Kawai M. has no conflict of interest. Maruko R received lecturing and travel fees from Kyowa Kirin. Koizumi H. received grants from Novartis Pharma, Alcon Pharma, Japan Alcon, Bayer Pharmaceuticals, HOYA, Senju Pharmaceutical, Santen Pharmaceutical, AMO, Otsuka Pharmaceutical, Star Japan, First Medical, Pfizer, Kowa, and Nikon; received lecturing and travel fees from Novartis Pharma, Alcon Pharma, Bayer Pharmaceuticals, Senju Pharmaceutical, Santen Pharmaceutical class, Kowa, Japan Alcon, HOYA, AMO, Otsuka Pharmaceutical, Pfizer, Bausch + Lomb Japan, JFC Sales Plan, Canon, Nidek, Abbott, Tomey, Daiichi Sankyo, Chugai Pharmaceutical, and Sanofi; and received consultant fee from Novartis Pharma, Bayer Pharmaceuticals, Chugai Pharmaceutical, Allergan, Japan Boehringer Ingelheim. Terao N. received lecturing and travel fees from Novartis Pharma, Bayer Pharmaceuticals, Santen Pharmaceutical, Abbott, Kowa, Topcon, Asahi Kasei Pharma, HOYA, and Chugai Pharmaceutical. Wakugawa S. received lecturing and travel fees from Senju Pharmaceutical and Novartis Pharma. Miyara Y. received lecturing and travel fees from Senju Pharmaceutical. Okada A. A. received grants from Alcon Pharma, Bayer Pharmaceuticals, Mitsubishi Tanabe Pharma, Pfizer, and Santen; received consultant fee from Apellis, Bayer Consumer Care AG, Bayer Pharmaceuticals, Biocon Biologics, Chugai Pharmaceutical, and Kowa; and received lecturing and travel fees from Bayer Australia, Bayer Pharmaceuticals, Chugai Pharmaceutical, Kowa, Mitsubishi Tanabe Pharma, Novartis Pharma, Santen, and Senju Pharmaceutical. Kataoka K. received lecturing and travel fees from Santen Pharmaceutical, Bayer Pharmaceuticals, Senju Pharmaceutical, Japan Boehringer Ingelheim, Otsuka Pharmaceutical, Canon Medtech, OMC Chugai Pharmaceutical, and Novartis Pharma. Yamamoto A. received lecturing and travel fees from Novartis Pharma, Bayer Pharmaceuticals, and Chugai Pharmaceutical. Nakayama M. has no conflict of interest. Ishiryu T. received grants from Novartis Pharma, Pfizer, Japan Alcon, Wakamoto Pharmaceutical, HOYA, Bayer Pharmaceuticals, Santen Pharmaceutical, Senju Pharmaceutical, AMO Japan, and Kowa; received lecturing and travel fees from Novartis Pharma, Chugai Pharmaceutical, Akura, Abbott, Alcon Pharma, Santen Pharmaceutical, and Senju Pharmaceutical; and received consultant fee from Allergan Japan. Mukai R. received lecturing and travel fees from Alcon Pharma, Chugai Pharmaceutical, and Senju Pharmaceutical. Itagaki K. received lecturing and travel fees from Novartis Pharma, Bayer, Santen Pharmaceutical, Senju Pharmaceutical, and Chugai Pharmaceutical. Ogasawara M. and Honjo J. have no conflict of interest. Mori R. received lecturing and travel fees from Santen Pharmaceutical, Bayer Pharmaceuticals, Japan Boehringer Ingelheim, and Kyowa Kirin and received lecturing and travel fees from Novartis Pharma, Senju Pharmaceutical, and Chugai Pharmaceutical. Tanaka K. received lecturing and travel fees from Santen Pharmaceutical, Alcon, Bayer Pharmaceuticals, Novartis Pharma, Senju Pharmaceutical, Nihon Tengan Kenkyusho, and Chugai Pharmaceutical. Wakatsuki Y. has no conflict of interest. Onoe M. received lecturing and travel fees from Novartis Pharma, Senju Pharmaceutical, and Chugai Pharmaceutical.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kataoka, K., Itagaki, K., Hashiya, N. et al. Six-month outcomes of switching from aflibercept to faricimab in refractory cases of neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 262, 43–51 (2024). https://doi.org/10.1007/s00417-023-06222-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06222-x